Background
The host immune response to bacterial dental plaque determines periodontal disease susceptibility by increasing the secretion of inflammatory cytokines. The Epidermal Growth Factor family cytokines stimulate proliferation and keratinization of cells in dermis and oral epithelium. Epidermal Growth Factor family consists of Amphiregulin, Betacellulin, Epiregulin, Epigen, Heparin binding Epidermal Growth Factor like growth factor and transforming Growth Factor-alpha.
Aim
The current study aimed to investigate expression of Betacellulin in chronic periodontitis patients with and without type 2 diabetes mellitus and thereby assessing role of betacellulin in periodontal health and disease.
Materials and Methods
Present study comprised of 90 participants, age ranges from 18 to 60-year-old, for the period of March 2010 to May 2011. Participants were categorized into three groups based Gingival index (GI), probing depth (PD) and clinical attachment loss (CA Loss). Group 1 consisted 30 individuals with clinically healthy periodontium, Group-2 consisted 30 individuals with GI>1, PD≥5 mm, and CA Loss>3 mm. Group-3 (Chronic Periodontitis with type 2 diabetes mellitus) consisted 30 with GI >1, PD≥5 mm, and CA Loss>3 mm. Immunohistochemical localization and quantification of Betacellulin was done in gingival tissue samples from all groups.
Results
Data showed expression of Betacellulin were higher in chronic periodontitis as compared to healthy. A positive correlation found in Betacellulin expression and Probing Depth in chronic periodontitis.
Conclusion
This footmark study impacts the role of Betacellulin in pathogenesis and progression of periodontal disease which will help in exploration of novel immunotherapeutic strategies and immunological research activity in this field.
Introduction
Gingival inflammation is regulated by reciprocal interactions between various cell types including, leukocytes, epithelial cells, fibroblasts and endothelial cells. These cell-cell interactions are mediated by complex interplay of soluble factors (cytokines) and matrix macromolecules. Epidermal Growth Factor (EGF) is one of the cytokines, which appears to contribute to inflammatory response, as well as numerous other physiological and pathological processes [1].
Members of EGF family show many important biological activities; particularly as potent mitogens, thus important in growth, development and tissue repair [2]. EGF signaling occurs through epidermal growth factor receptor (ErbB) receptor tyrosine kinases [3]. Betacellulin (BTC) a new member of epidermal growth factor family was originally isolated from insulinoma cell line and is located on chromosome 4q13-q21 [4]. BTC is predominantly found in the pancreas and intestine and plays a role in their regulation and/or differentiation. It was shown that BTC stimulate regeneration of pancreatic Beta–cells and promote growth of cytokeratin-positive ductal cells and also increase the number of islet like cell clusters [5].
BTC can bind to epidermal growth factor receptor, ErbB4 to form ErbB1/ErbB4 heterodimers. Once bound by homo or heterodimerized ErbB receptors, it initiates multiple signaling pathways that affect proliferation, survival, and differentiation that are mediated by Ras-Raf-mitogen-activated protein kinase (MAPK).
The MAPK family of protein kinases includes the extracellular signal-regulated kinases (ERKs), the c-Jun N-terminal kinases/stress activated protein kinases (JNK/SAPK). Within the MAPK family, the ERK and JNK pathways have been reported to be stimulated by receptor tyrosine kinases in various cell types. Many growth factors have been reported to stimulate keratinocyte migration and it has been shown that receptor tyrosine kinase and integrin- induced cell migration involve activation of the Ras/MAPK signal transuduction pathway. Similarly, receptor tyrosine ligands induce a number of extracellular matrix degrading proteases including MMP-9, and ERK, JNK and MAPK pathways have been reported to contribute to MMP-9 gene expression. Studies suggested that prolonged activation of MAPK activity may be a general requirement for induction of MMP-9 expression [6].
It was clearly demonstrated in vivo, that periodontal ligament fibroblasts, preosteoblasts and prechondrocytes expressed numerous specific binding sites for EGF [7] and an up-regulation of cell surface receptors for EGF during the inflammatory response [1]. Aberrant expression of EGF receptor and one or more of its ligands may result in autocrine or paracrine activation. It can lead to marked induction of several extracellular degrading enzymes, matrix metalloproteinase -9 (MMP-9) in particular [8]. Evidence showed that BTC induces MMP-9 production and invasion primarily through activation of EGFR, MAPK and P13K/Akt in Head Neck Squamous Cell Carcinoma (HNSCC) [7]. MMP9 is produced by various cells in the oral cavity and its production is also elevated during periodontitis [9].
Betacellulin and epiregulin (another member of EGF Family) induced Prostaglandin E2 (PGE2) production by up-regulating cyclooxygenase-2 expression through ERK1/2 signaling in human granulosa cells [10]. There is enough evidence that PGE2 is an important mediator in the periodontal inflammation and bone destruction and also involved in tissue response regulation. Prostaglandin E2 plays an important role in periodontal inflammation by stimulating the suppression of lymphocyte production, decreasing the collagen synthesis by fibroblasts and influencing osteoclastic bone resorption [11,12].
Prostaglandin E2 and MMP-9 both are well known pro-inflammatory markers for periodontal disease and BTC induces upregulation of both of these markers. Scanty of literature is available regarding expression of BTC in oral cavity. To the best of our knowledge there are no studies concerning expression of BTC in periodontal diseases. Hence this initial research was undertaken to emphasize on BTC and periodontitis. The present study aimed to compare and quantify the expression levels of BTC in gingival tissues of subjects with healthy periodontium, chronic periodontitis patients and chronic periodontitis patients with type 2 diabetes mellitus and also to correlate the expression levels of betacellulin with the severity of periodontal disease, which would be helpful for diagnosis and implementation of novel therapeutic modalities.
Materials and Methods
Source of Data
The present study was carried out on 90 participants (47 males and 43 females), age ranging from 18 to 60-year-old, who visited the Outpatient Department of Periodontics and Department of Oral and Maxillofacial Surgery of PMNM Dental College and Hospital at Bagalkot, during March 2010 to May 2011.
Methods of Collection of Data
The protocol of the study was in accordance with the Helsinki Declaration of 1975, as revised in 2002, approved by the Institutional ethical committee of Dental College and Hospital. The protocol was clearly explained to all potential participants and written consent was obtained. A proforma was designed to record relevant data comprising of details of the chief complaint, preliminary history etc, and clinical parameters as gingival index (GI) [13], probing depth (PD) and clinical attachment loss (CA Loss) [14,15]. Probing depth and CA loss measurements were taken using William’s periodontal probe and were taken at four sites (i.e. mesial, distal, mid buccal, and mid palatal) around each tooth.
Gingival tissue samples collected from all the 90 subjects were categorized into three groups based on their clinical findings as follows:
Group 1 – 30 gingival tissue samples from healthy controls without periodontitis or any other systemic disease.
Group-2 – 30 gingival tissue samples from chronic periodontitis patients without any other systemic disease.
Group-3 – 30 gingival tissue samples from chronic periodontitis patients with type 2 diabetes mellitus.
Inclusion criteria for group-1 is healthy gingival condition with gingival index score<1 and no sites with probing depth >4mm or clinical attachment loss >1mm. Group-2 consisted of untreated chronic periodontitis with a probing depth >5mm and clinical attachment loss ≥3mm. Patients with Type 2 diabetes who had good glycaemic control (Hb A1C<8%) [16], untreated chronic periodontitis with a probing depth >5mm and Clinical attachment loss ≥3mm were included in group-3.
Active smokers, immunocompromised patients and those who have history of periodontal treatment in past 6 months were excluded from the study. Also, patients under antibiotic treatment during past 6 months, pregnant women along with lactating mothers and patients with gross oral pathology or tumours, with aggressive periodontitis were excluded from study. Diabetic patients were excluded in Group 1 and 2. Uncontrolled diabetic patients were excluded in Group-3.
Specimen Collection
Gingival tissue samples for group-1were obtained during extraction of teeth for the purpose of orthodontic treatment and third molar extraction. Gingival specimens for group-2 and group-3 were obtained from the periodontitis patients indicated for periodontal surgery undergoing modified flap operation after completion of scaling and root planning. Gingival biopsies harvested using Bard Parker handle no.3 and no.15 blade from either Buccal or lingual site. While obtaining gingival tissues from group-2 and group-3 it was made sure that biopsies should include pocket epithelium. A Tissue of 5mmx3mm was excised and were fixed in 4% buffered formalin and transported to the laboratory.
Immunohistochemistry (IHC) Procedure
After processing, the specimens were embedded in paraffin wax and cut in thin sections of 4μm by using a semi-automatic microtome and mounted on glass slides and air dried at 56°C for 20min. These sections were dewaxed in xylene and placed in decreasing concentration of alcohol for rehydration. Antigen retrieval was carried out by immersing the slides in citrate buffer and incubating in enzyme retriever for 95°C for 10 minutes. Then the primary antibody Human Betacellulin Affinity purified Polyclonal antibody (1μg/ml) was added and incubated 20°C for 1hour. The Antibody was diluted in 1 ml of sterile Phosphate Buffer Solution (PBS) followed by 100μl of slock in 600μl PBS. The secondary antibody staining was done. Briefly, these sections were incubated for 30 min in Super enhancer reagent and rinsed well with buffer. This procedure was followed by application of Polymer Horse raddish Peroxidase (HRP) reagent for 15 minutes and rinsed with buffer. Then substrate solution was applied on tissue sections for 10 minutes and labelled with the Di Amino Benzedine chromogen (DAB) for 10 minutes and were counter stained with Meyer’s Haematoxylin for 5 to 10 minutes, followed by rinsing with tap water. Lastly the sections were immersed in ammonia water for 10sec, mounted and analysed.
Betacellulin Analysis
Stained slides were first screened and the area for examination was determined by two independent observers, using a light microscope equipped with a digital video camera at the Department of Oral Pathology, in a Institute of Dental Sciences and Research Centre. Each slide was analysed for the intensity of Betacellulin positive stained cells in the epithelium. The level of intensity of Betacellulin expression in the epithelium was quantified in semi-quantitative method. Grading [17] was done in following manner using a 0 to +3 scales: 0 as no staining; +1 as weak staining comprising basal layer of epithelium (Mild); +2 as moderate staining comprising basal and suprabasal layer of epithelium; +3 as strong stain comprising all layers with high intensity.
Statistical Analysis
Data obtained were statistically analysed using Statistica 6.0 system. A Kruskal Wallis analysis and Chi-square test were carried out for a comparison of Betacellulin levels among groups. Spearman’s correlation coefficient (p=0.05) was used to correlate between betacellulin concentration and clinical parameters.
Results
The immunohistochemical evaluation results by two independent observers were subjected to inter-examiner calibration by calculating the κ-score. The κ-score was almost 96.67% and therefore, indicated an ‘almost perfect’ strength of inter-examiner agreement beyond chance (p<0.05). In [Table/Fig-1], healthy group-3/30 tissue samples showed expression of BTC. In chronic periodontitis group 11/30 tissue samples stained positive for BTC and in Chronic periodontitis with type 2 diabetes mellitus group 9/30 tissue samples expressed BTC.
Distribution of study population and intensity categories with the Kruskal-Wallis Test
| Group | Negative | % | Mildpositive | % | Moderatepositive | % | Severelypositive | % | Total |
|---|
| Healthy subjects (Group 1) | 27 | 90.00 | 2 | 6.67 | 1 | 3.33 | 0 | 0.00 | 30 |
| Chronic periodontits (Group-2) | 19 | 63.33 | 8 | 26.67 | 1 | 3.33 | 2 | 6.67 | 30 |
| Chronic periodontits with diabetes (Group-3) | 21 | 70.00 | 6 | 20.00 | 3 | 10.00 | 0 | 0.00 | 30 |
| Total | 67 | 74.44 | 16 | 17.78 | 5 | 5.56 | 2 | 2.22 | 90 |
p-value >0.05
[Table/Fig-2] shows comparison of expression of BTC among three groups. Comparison between Group 1 and Group-2 portrayed statistically significant difference; whereas Group 1 and group-3 showed no statistical significant difference. Also, the expression of BTC among Group-2 and Group-3 was statistically non-significant. Thus the [Table/Fig-2] depicts that expression of BTC was higher in chronic periodontitis group than other two groups.
Comparison of Betacellulin expression among the study groups with Chi-Square Test
| Groups | Chi-Square Value | p-value |
|---|
| Healthy subjects (Group1) vschronic periodontitis (Group2) | Chi-square= 8.0206 df=3 | p=0.0455*, S |
| Healthy subjects (Group1) vschronic periodontitis with type 2diabetes (Group3) | Chi-square= 3.750 df=2 | p=0.1533, NS |
| Chronic periodontitis (Group2) vschronic periodontitis with type 2diabetes (Group3) | Chi-square= 3.3860 df=3 | p=0.3359, NS |
*Statistically significant at p-value <0.05
In [Table/Fig-3] BTC expressions were correlated with GI and clinical parameters (PD and CA Loss). The correlation between BTC and GI score was weak and negative in healthy group. In chronic periodontitis group, the correlation between BTC and GI was weak but positive and not statistically significant. A significant positive correlation found between BTC expression and PD in chronic periodontitis group. The correlation between BTC and CA Loss was weakly positive but not statistically significant. In chronic periodontitis with diabetes mellitus group, the correlation between BTC and GI and CA Loss was positive but was not statistically significant. A positive correlation was seen between PD and BTC expression but was not statistically significant [Table/Fig-3].
Spearman’s rank correlation Test to compare Betacellulin expression with GI, PD and CAL among three groups
| Groups | Parameters | Rank correlationcoefficient | p-value |
|---|
| Healthy subjects (Group 1) | GI | 0.0626 | 0.7424 |
| PD | 0.0000 | 1.0000 |
| CAL | — | — |
| Chronic periodontitis (Group-2) | GI | 0.1624 | 0.3827 |
| PD | 0.694 | 0.0426* |
| CAL | 0.128 | 0.501 |
| Chronic periodontitis with type 2 diabetes (Group-3) | GI | 0.0155 | 0.9340 |
| PD | -0.0803 | 0.6675 |
| CAL | 0.2414 | 0.1909 |
* Statistically significant; —not applicable
Discussion
Periodontal diseases are mediated by the interaction between pathogens and host immunoinflammatory responses. Since the inflammatory mediators can profoundly affect the protective or destructive nature of the host response, there has been increasing interest in identifying the role played by cellular and molecular factors in the pathogenesis of periodontal diseases. Being an important member of EGF family, Betacellulin plays a vital role in inflammation and tissue repair.
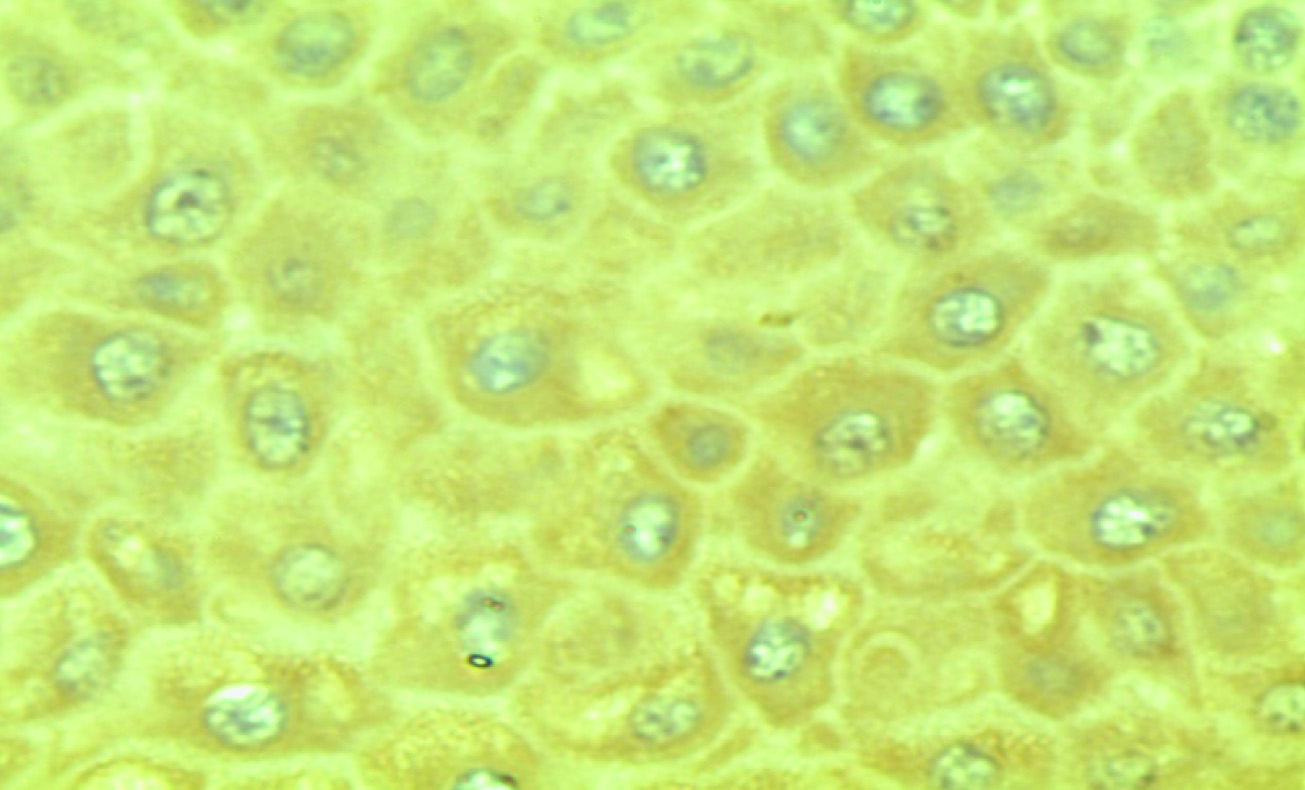
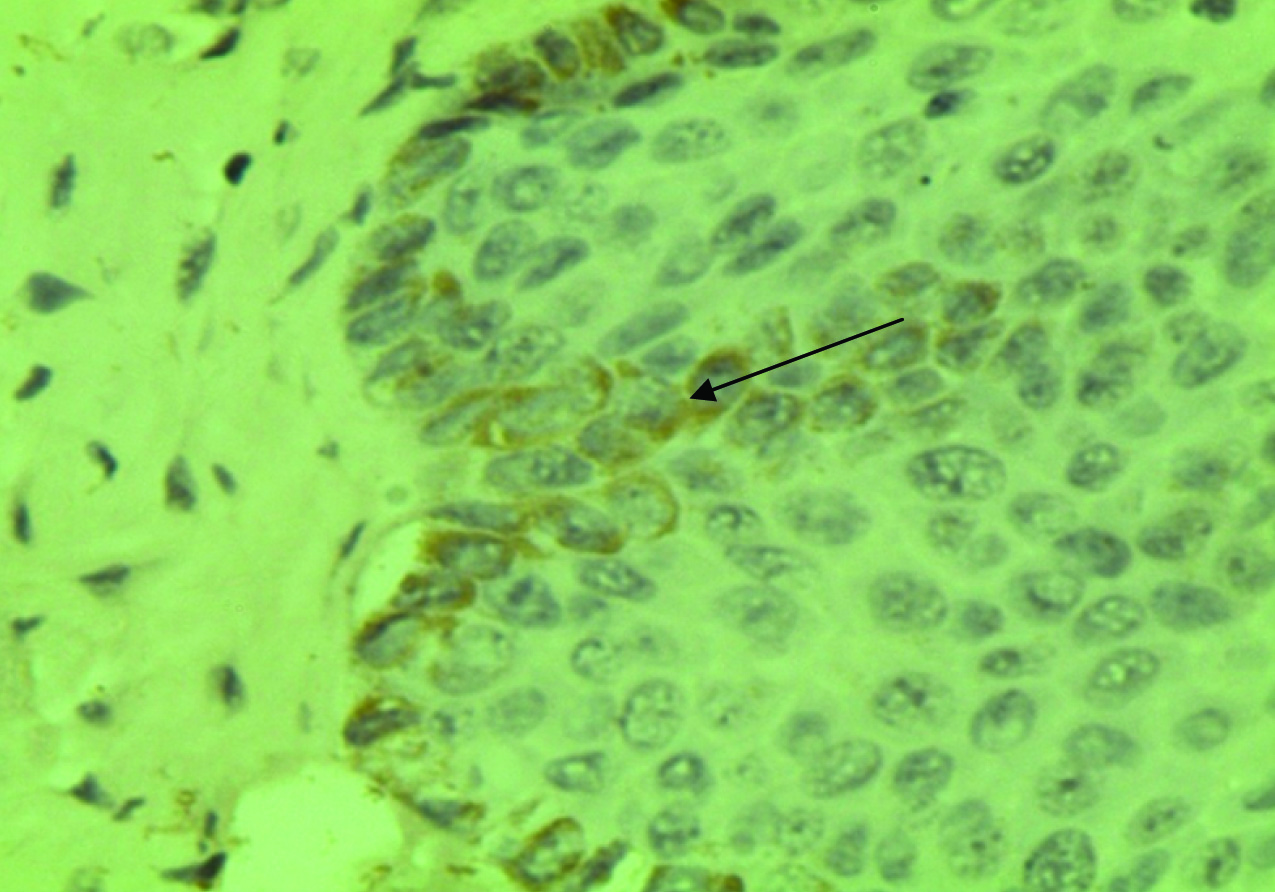
The current immunohistochemical research was undertaken to find out the expression of BTC and its role in gingival health and disease. Betacellulin specific staining appeared as brown stain of varying intensity, especially on the transmembrane of keratinocytes present in basal cell layer of gingival epithelium. [Table/Fig-4] showed staining pattern of Betacellulin in all layers of epithelium in Group-2 and [Table/Fig-5] showed betacellulin staining confined to basal layer of epithelium. Our study compared three groups (healthy, chronic periodontitis, chronic periodontitis with diabetes mellitus). The results of our study show that BTC expression was less in healthy group as compared with chronic periodontitis and chronic periodontitis with diabetes mellitus. This indicates that expression of BTC is increased in inflamed tissue than that of healthy tissue. The variability in appearance of BTC in healthy and chronic periodontitis group could be because of different stages of disease process. Though some specimens from healthy group showed positive staining for BTC, reason could be mild inflammatory alterations present at the time of tissue excision. Betacellulin staining pattern in chronic periodontitis with diabetes mellitus group is similar to chronic periodontitis group which may be because of good glycaemic control in Group-3.
Photomicrograph showing brown coloured Betacellulin staining in all layers of epithelium in gingival tissue in chronic periodontitis group
Photomicrograph showing brown coloured Betacellulin staining confined to basal layer in chronic periodontitis with type 2 diabetes mellitus group
Overall result of the present study suggests that, the expression of BTC was higher in chronic periodontitis group as compared with healthy controls which was statistically significant. Moreover, BTC expression increased with the increasing severity of periodontal disease. Betacellulin expression was somewhat elevated in chronic periodontitis group as compared to chronic periodontitis patients with type 2 diabetes mellitus group but the difference was not significant.
According available literature, no study has been conducted to find out the association of BTC with periodontal diseases. So far, studies have been conducted on localization of BTC in other parts of body [18], and in various carcinomas. However some previous immunohistochemical studies have find out expression of EGFR in oral mucosa and gingiva.
It can be speculated from our results that BTC specific staining was less in the epithelium of histologically healthy gingiva and its expression increased in inflamed tissue. Similar findings were reported by Bergler et al., [19], who failed to detect the EGF-receptor in normal cheek mucosa of 10/12 patients. However, Whitcomb et al., [20] found expression for EGFR in normal tissues from eight sites of human oral mucosa. Moreover, other investigators have also reported the presence of EGF receptors on epithelial cells of normal oral mucosa, including gingiva.
Expression of BTC was increased in group-2 and group-3, however disparity in the number and intensity of BTC specific staining among Group-2 and Group-3 may be because of variation in host tissue response. Similar results were reported by Irwin et al and Nordlund et al, where they demonstrated increased levels of EGF receptors in inflamed gingiva and in adult periodontitis [1,21]. They observed an increase in the expression of cell surface receptor in inflamed tissue, but the mechanism responsible for an up regulation in receptor expression was not known.
Various studies investigated that BTC upregulates MMP-9 production in HNSCC through activation of EGFR and MAPK [8,22]. A study suggested that MMP-9 levels in the gingival crevicular fluid are higher in chronic periodontitis group than in patients with gingivitis and healthy subjects [23]. Similarly another study demonstrated an increased concentration of MMP-9 in the gingival tissue of diabetic chronic periodontitis patients suggests that the expression of these MMPs contributes to the failure of the healing process in the diabetic condition [24]. Prostaglandin E2 and MMP-9 both are well known pro-inflammatory markers for periodontal disease and BTC induces upregulation of both of these markers. So, the expression of BTC in gingival tissue can be considered an inflammatory marker in periodontal disease.
As various studies have shown that BTC induces production of MMP-9 which can cause destruction of extracellular matrix and basement membrane and also leads to initiation and progression of periodontal disease. BTC also upregulates PGE-2 production which is a known proinflammatory biomarker for progression of periodontal disease. The role of MMP-9 and PGE2 in the destructive processes of periodontal disease has been proved, distinguishing them as a viable target for a chemotherapeutic approach. The use of BTC inhibitor along with Tissue inhibitor matrix metallo proteinases and antinflammatory agents as an adjunct to conventional periodontal treatment can enhance and make clinical therapeutic responses more predictable.
One objective of periodontal research is to develop diagnostic tests to detect metabolic alterations that occur in the initial phase of periodontal disease which would be helpful in preventing further progression of disease. In future, studies can be done to confirm the role of BTC as a biomarker for periodontal disease by using gingival crevicular fluid and saliva with various other diagnostic techniques.
Limitations
Within the limitations of the study, some errors can be suspected in laboratory procedures as they are technically very sensitive. So there is a necessity to carry out further prospective studies with large sample size and with different refined techniques to validate the role of BTC in pathogenesis of periodontitis and to affirm the observations of our study.
Conclusion
From results of the present study, it can be concluded that BTC expression is associated with chronic periodontitis. Further studies with advanced molecular level research are required to establish the association of BTC in the pathogenesis and progression of periodontal disease which in turn may help in exploration of novel immunotherapeutic strategies. Hence this would be the footmark study which will definitely have an impact for future immunological research activity in this field.
p-value >0.05
*Statistically significant at p-value <0.05
* Statistically significant; —not applicable
[1]. Irwin CR, Schor SL, Ferguson MWJ, Expression of EGF-receptors on epithelial and stromal cells of normal and inflamed gingivaJ Periodontol 1991 26:388-94. [Google Scholar]
[2]. Mummery SR, Mulloy B, Rider CC, The binding of human betacellulin to heparin, heparan sulfate and related polysaccharidesGlycobiol 2007 17(10):1094-1103. [Google Scholar]
[3]. Genetos DC, Rao RR, Vidal MA, Betacellulin inhibits osteogenic differentiation and stimulates proliferation through HIF-1 alphaCell tissue res 2010 340:81-89. [Google Scholar]
[4]. Elbein SC, Wang X, Karim MA, Chu WS, Silver KD, Analysis of coding variants in the betacellulin gene in type 2 diabetes and insulin secretion in African American subjectsBMC Medical Genetics 2006 7:62 [Google Scholar]
[5]. Li L, Seno M, Yamada H, Kojama I, Betacellulin improves glucose metabolism by promoting conversion of intra islet precursor cells to B-cells in streptozotocin-treated miceAm J Physiol Endocrinol 2003 285:577-83. [Google Scholar]
[6]. McCawley LJ, LI S, Elizabeth V, Wattenberg Hudson LG, Sustained activation of the mitogen-activated protein kinase pathwayJ Biol Chem 1999 271:1317-53. [Google Scholar]
[7]. Cho M-I, Garant PR, Lee YL, Periodontal ligament fibroblasts, preosteoblasts, and prechondrocytes express receptors for epidermal growth factor in vivo: A comparative radioautographic studyJ Periodontal Res 1988 23:287-94. [Google Scholar]
[8]. Pornchai OC, Wongkajomsilp A, Rhys Evas HP, Eccles AS, Signaling pathways required for matrix metalloproteinase-9 induction by betacellulin in head and neck squamous carcinoma cellsInter J cancer 2004 111(2):174-83. [Google Scholar]
[9]. Makela M, Salo T, Uitto VJ, Larjava H, Matrix metalloprotienases (mmp-2 and mmp-9) of the oral cavity: cellular origin and relationship to periodontal statusJ Dent Res 1994 73(8):1397-406. [Google Scholar]
[10]. Fang L, Cheng JC, Chang HM, Sun YP, Leung PC, EGF-like growth factors induce COX-2-derived PGE2 production through ERK1/2 in human granulosa cellsJ Clin Endocrinol Metab 2013 98(12):4932-41.[Epub ahead of print] [Google Scholar]
[11]. Tipton A, Flin J, Dabbous M, Cyclooxygenase - 2 inhibitors decrease interleukin- 1â –stimulatet prostaglandin E2 and IL-6 production by human gingival fibroblastsJ Periodontol 2003 74:1754-63. [Google Scholar]
[12]. Vardar-Sengûl S, Baylas H, Huseyinov A, Effect of selective cyclooxygenase-2 inhibition on gingival tissue levels of prostaglandin E2 and prostaglandin F2á and clinical parameters of chronic periodontitisJ Periodontol 2003 74:57-63. [Google Scholar]
[13]. Loe H, Silness J, Periodontal disease in pregnancy. I. Prevalence and severityActa Odontol Scand 1963 21:533-51. [Google Scholar]
[14]. Mariano S, Michael G, Newman Marc Q, Clinical diagnosis. In Newman MG, Takei HH, Klokkevold PR, Carranza FAClinical Periodontology 2007 10th EditionIndiaWB Saunders Co:540-60. [Google Scholar]
[15]. Sture N, Jan Lindhe, Examination of patients with periodontal disease. In Jan Lindhe, Thorkild K, and Niklaus P. LangClinical Periodontology and Implant Dentistry 2003 4th EditionIndiaGopsons papers Ltd:403-13. [Google Scholar]
[16]. Brian L, Mealey Klokkevold PR, Otomo-Corgel J, Periodontal treatment of medically compromised patients. In Newman MG, Takei HH, Klokkevold PR, Carranza FAClinical Periodontology 2007 10th EditionIndiaWB Saunders Co:650-674. [Google Scholar]
[17]. Kotsovilis S, Tseleni-Balafouta S, Charonis A, Fourmousis I, Nikolidakis D, Vrotsos JA, Syndecan-1 immunohistochemical expression in gingival tissues of chronic periodontitis patients correlated with various putative factorsJ Periodont Res 2010 45:520-31. [Google Scholar]
[18]. Miyagwa J, Hanafusa T, Sasada R, Yamamoto K, Igarshi K, Yamamori K, Immunohistochemical localization of Betacellulin, a new member of EGF family, in normal human pancreas and islet tumour cellsEndocr J 1999 46(6):755-64. [Google Scholar]
[19]. Bergler W, Bier H, Ganzer The expression of epidermal growth factor receptors in the oral mucosa of patients with oral cancerArch Otorhinolaryngol 1989 246:121-25. [Google Scholar]
[20]. Whitcomb SS, Eversole LR, Lindemann RA, Immunohistochemical mapping of epidermal growth-factor receptors in normal human oral soft tissueArch Oral Biol 1993 38(9):823-26. [Google Scholar]
[21]. Nordlund L, Hormia M, Saxen L, Thesleff I, Immunohistochemical localization of epidermal factor receptors in human gingival epitheliaJ Periodontal Res 1991 26:333-38. [Google Scholar]
[22]. Kondapaka BS, Fridman R, Reddy BK, Epidermal growth factor and Amphiregulin up-regulate matrix metalloproteinase -9 (MMP-9) in human breast cancer cellsInt J Cancer 1997 70:722-26. [Google Scholar]
[23]. Maeso G, Bravo M, Baseones A, Levels of metalloproteinase-2 and-9 and tissue inhibitor of matrix metalloproteinase -1 in gingival crevicular fluid of patients with periodontitis, gingivitis, and healthy gingivalQuintessence Int 2007 38:247-52. [Google Scholar]
[24]. Kumar MS, Vamsi G, Sripriya R, Sehgal PK, Expression of matrix metalloproteinases (MMP-8 and -9) in chronic periodontitis patients with and without diabetes mellitusJ Periodontol 2006 77(11):1803-08. [Google Scholar]