Adverse drug reaction (ADR) has been defined by the World Health Organization (WHO) as “any response to a drug which is noxious and unintended and which occurs at doses normally used in man for prophylaxis, diagnosis, or therapy of disease, or for the modification of physiological function” [1]. A meta analysis of 39 epidemiological studies by Lazarou et al., found that ADRs ranked fourth and sixth leading causes of deaths in USA [2]. Considering the importance of monitoring ADRs to improve public health, Pharmacovigilance programme of India (PvPI) was started in 2010 [3]. As per this program, ADR monitoring centers have been started in many medical institutions all over the country to estimate the frequency of ADRs occurring with various drugs among the Indians.
Cardiovascular diseases (CVD), one of the most common causes of morbidity and mortality worldwide is estimated to increase from 16.7 million to 23.3 million by 2030 [4–6]. On par with the global burden, CVDs constitute the leading cause of deaths among the non-communicable diseases in India [7]. Since the prevalence of CVD is on the rise, the number of patients prescribed with cardiovascular drugs is also escalating. In addition as patients with CVD are prescribed multiple drugs compared to other diseases, there is an accentuation of ADRs due to polypharmacy [8–10]. This has been further confirmed in a study by Lesar et al., which found ADRs with cardiovascular drugs to be 2.4 times higher, compared to other drugs [11]. Thus cardiovascular drugs one of the most commonly prescribed drugs in the hospital and also prone for medication errors needs to be monitored for the ADRs associated with them [12]. However, the pattern of occurrence of ADRs with cardiovascular drugs are limited in India [9,10]. Hence the present study aims to analyse ADRs reported with cardiovascular drugs in a tertiary care teaching hospital.
Materials and Methods
This study analysed the ADRs of cardiovascular drugs that were reported to ADR monitoring centre (AMC), functioning from Department of Clinical Pharmacology, JIPMER by spontaneous reporting and active surveillance methods. The total study period was 27 months from January 2011 to March 2013. During this period all the ADRs caused by cardiovascular drugs reported to the AMC was included for the study.
Spontaneous reporting of ADRs voluntarily by the healthcare professionals has been the core data-generating system of pharmacovigilance for years. It plays a major role in identifying and reporting of any adverse events to the pharmacovigilance coordinating centre, health/regulatory authority or to the drug manufacturer itself [13]. However, active surveillance that includes dynamic searching for exposures and health outcomes plays a major role in detecting newer and rarer ADRs within a shorter time span [14]. Hence it was decided to include ADRs reported through both spontaneous reporting and active surveillance for analysis of ADRs caused by cardiovascular drugs in the present study.
The ADRs reported to AMC were analysed by pharmacovigilance team comprising of Clinical Pharmacologists and a drug technical associate working under PvPI. The causality analysis of the reported ADR was done using WHO Causality Assessment Scale and they were classified as certain, probable, possible, unlikely, unclassified as well as unclassifiable [1]. Causality of ADRs analysed by Naranjo’s scale was graded as definite, probable, possible and doubtful [15]. The severity of ADRs was analysed using modified Hartwig Siegel’s severity assessment scale as mild, moderate and severe [16].
Results
During the study period, a total of 2188 ADR reports were received and of these cardiovascular drug related ADRs were 397 (18.1%). A total of 397 patients had developed 463 ADRs during the entire study period and among them 319 patients were males and 78 patients were females as shown in [Table/Fig-1]. The age of the patients ranged from 11 to 91 years, with majority of the patients belonging to the age group of 31–59 years followed by elderly patients aged 60 years and above as well as patients aged 30 years and below. Among the ADRs 98% reports were collected by active surveillance method while only 2% were received through spontaneous reporting system.
| Parameters studied | Sample size (N=397) |
|---|
| Age (%) | < 30 years (3.3) |
| 31 – 59 years (51.1) |
| > 60 years (45.6) |
| Male: Female (%) | 319 (80.4):78 (19.6) |
| No of ADRs reported | 463 |
Classification of ADRs by common terminology criteria for adverse events (CTCAE ver. 4.0) [17] showed that most of the ADRs manifested as gastrointestinal system disorder (20.7%) comprising of gastritis, constipation and gastrointestinal bleeding. This was followed by respiratory system disorders (18.4%) and nervous system ADRs (15.1%). Respiratory system disorders included cough and dyspnea while nervous system ADRs comprised of headache and giddiness. The involvement of other organ systems in the adverse drug reactions are given in [Table/Fig-2].
Classification of ADRs by CTCAE v 4.0
| Organ – System involved | No. ofADRs | Percentage (95% CI)N=463 |
|---|
| Gastrointestinal disorders | 96 | 20.7 (17 – 24.4) |
| Respiratory, thoracic and mediastinal disorders | 85 | 18.4 (14.8 – 21.9) |
| Nervous system disorders | 70 | 15.1 (11.9 – 18.4) |
| General disorders and administration site conditions | 58 | 12.5 (9.5 – 15.5) |
| Musculoskeletal and connective tissue disorders | 48 | 10.4 (7.5 – 13.1) |
| Psychiatric disorders | 22 | 4.8 (3 – 7.1) |
| Metabolism and nutritional disorders | 21 | 4.5 (2.8 – 6.8) |
| Cardiac disorders | 19 | 4.1 (2.4 – 6.3) |
| Skin and subcutaneous tissue disorders | 17 | 3.7 (2.1 – 5.8) |
| Others | 14 | 3 (1.6 - 5) |
| Reproductive system and breast disorders | 13 | 2.8 (1.5 – 4.7) |
Others include Immune system disorders, Endocrine disorders, Eye disorders, Renal and urinary disorders, Blood and lymphatic system disorders, Ear and labyrinth disorders, Hepatobiliary disorders
ADR- adverse drug reaction CTCAE: Common Terminology Criteria for Adverse Events
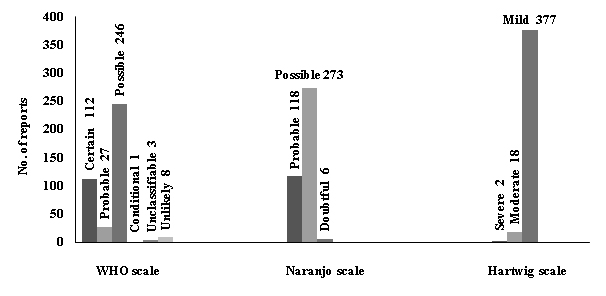
The top 10 drugs associated with ADRs were shown in [Table/Fig-3]. Enalapril was the most commonly implicated drug in the study population with 81 (17.5%) ADRs. The other common drugs involved in causing ADRs included atorvastatin, aspirin, metoprolol and atenolol. According to the WHO causality assessment most of the adverse drug reactions fell in the category of “possible” (62%) followed by “certain” (28.2%) and “probable” (6.8%). Similarly analysis with Naranjo scale revealed that majority of the ADRs were possible (68.8%) followed by probable (29.7%). This could be attributed to the differences in assessing methods of the scales, as the former is more subjective and the later is more objective. In addition Hartwig severity scale used to assess the severity of ADRs reported, showed that majority of the reports were of mild nature (95%) followed by moderate (4.5 %) as shown in [Table/Fig-4].
Top 10 drugs associated with occurrence of more ADRs in the present study
| Drug | No. ofADRs | Percentage (95% CI)N= 463 | Most frequent ADRs (N) |
|---|
| Enalapril | 81 | 17.5 (14 - 21) | Cough (77) |
| Angioedema (2) |
| Atorvastatin | 69 | 14.9 (11.7 – 18.1) | Myalgia (22) |
| Constipation (6) |
| Arthralgia (4) |
| Dry skin (4) |
| Aspirin | 39 | 8.4 (6 – 11.3) | Gastritis (23) |
| Tinnitus (2) |
| Malena (2) |
| Metoprolol | 39 | 8.4 (6 – 11.3) | Fatigue (13) |
| Insomnia (6) |
| Giddiness (6) |
| Atenolol | 37 | 7.9 (5.7 – 10.8) | Fatigue (10) |
| Giddiness (6) |
| Insomnia (5) |
| Ranolazine | 33 | 7.1 (4.9 – 9.8) | Palpitation (4) |
| Arthralgia (4) |
| Constipation (3) |
| Amlodipine | 20 | 4.3 (2.6 – 6.6) | Pedal edema (14) |
| Facial edema (3) |
| Isosorbidemononitrate | 19 | 4.1 (2.5 – 6.3) | Headache (18) |
| Dizziness (1) |
| Trimetazidine | 12 | 2.6 (1.3 – 4.5) | Constipation (3) |
| Weakness (3) |
| Abdominal pain (2) |
| Spironolactone | 9 | 1.9 (0.8 – 3.6) | Gynecomastia (8) |
| Metabolic alkalosis (1) |
ADR- adverse drug reaction
Assessment of ADRs using various scales
ADR- adverse drug reaction
Dry cough was the most frequently reported ADR with a frequency of 17.3%, followed by gastritis (7.5%), fatigue and myalgia (6.5%) as shown in [Table/Fig-5]. During the study period one patient died (0.2%) due to breathlessness following streptokinase administration. The rare adverse reactions reported during the study period included metoprolol-induced carpal tunnel syndrome, atenolol-induced heart failure and vivid dreams, atorvastatin-induced icthyosis, propranolol-induced somniloquy, amiodarone-induced thyroiditis, streptokinase-induced fatal breathlessness, atorvastatin-induced hepatic dysfunction and metoprolol-induced memory loss.
More frequent ADRs reported
| Major ADR | No. of ADRs | Percentage (95% CI)N= 463 |
|---|
| Cough | 80 | 17.3 (13.8 – 20.7) |
| Gastritis | 35 | 7.5 (5.3 – 10.4) |
| Fatigue | 30 | 6.5 (4.4 – 9.1) |
| Myalgia | 30 | 6.5 (4.4 – 9.1) |
| Headache | 25 | 5.4 (3.5 – 7.8) |
| Giddiness | 21 | 4.5 (2.8 – 6.8) |
| Insomnia | 17 | 3.6 (2.1 – 5.8) |
| Dizziness | 15 | 3.2 (1.8 – 5.3) |
| Pedal edema | 15 | 3.2 (1.8 – 5.3 |
| Constipation | 12 | 2.6 (1.3 – 4.5) |
ADR- adverse drug reaction
Discussion
The present study was done to evaluate the pattern of ADRs among the patients with CVD. Present study found that gastrointestinal (GI) and respiratory systems were most commonly affected by ADRs. The most frequently reported ADRs were dry cough and gastritis and the cardiovascular drugs implicated in causing these ADRs were found to be enalapril, atorvastatin and aspirin. This was in contrary to the findings of the previous Indian studies conducted to evaluate ADRs caused by cardiovascular drugs [9,10,18,19]. The study by Singhal et al., included 148 patients with 231 ADRs and concluded central nervous system (CNS) and GI system as the most frequent organs affected by ADR [18]. As per that study the most commonly reported ADRs were headache (24.2%) and dry cough (13.9%) while the common drugs causing ADRs were calcium channel blockers (23.4%) and nitrates (16.5%) [18]. Another study by Sharminder et al., has reported 138 ADRs from 188 patients subsequent to the use of cardiovascular drugs. In that study the most common ADRs were hypersensitivity skin reactions and headache, while most commonly involved drugs were nitrates (17.8%) and diuretics (11.5%) [10]. One more study conducted to evaluate the occurrence of ADRs following the use of cardiovascular drugs, found ADRs occurring as oral manifestations in 67.4% of the patients. In that study xerostomia or dryness of mouth was found to be the most common ADR (25.5%), followed by dysgeusia (17.7%), and a combination of both xerostomia and dysgeusia (12.4%) [19]. This could be attributed to the differences in the methodology, objectives as well as prescribing patterns among the previous studies compared to that of present study. The present study had less adverse effects with nitrates as opposed to high prevalence of nitrate induced adverse reactions from an earlier study conducted in North Indian population [10]. This difference could be due to ethnic variations among North Indians and South Indians in their response to nitrates but this hypothesis need to be confirmed by further studies.
A study by Gholami et al., found that Central nervous system and Gastrointestinal system disorders were the most frequent system-organ classes affected with ADRs. In the same study headache, vertigo, weakness etc were found to be the most frequent adverse reactions [20]. Likewise, present study found similar reactions in GI, respiratory and nervous systems. A study by Teweleit et al., found that the most often observed ADR were arrhythmias (27.1%), syncope and variations in blood pressure (25.1%). The drugs most frequently related to ADR were angiotensin converting enzyme (ACE) inhibitors (17.9%) and digitalis (17.3%) [21]. Present study had similar finding with enalapril (17.5%) causing more ADRs. A comparison of present study findings with other studies are shown in [Table/Fig-6].
Comparison of present study with other studies done to evaluate ADRs occurring with cardiovascular drugs
| Study by | Country | No. ofADRs | Systemaffected | CommonestADR | Commonlyimplicated drugs |
|---|
| Teweleit et al., 2001[21] | German | 559 | - | Arrhythmias, syncopes and blood pressure dysregulations | Angiotensin inhibitors, Digitalis |
| Zaidenstein et al., 2002 [22] | Israel | 20 | - | Orthostatic hypotension, bleeding, arrhythmias | Warfarin, Beta-blockers |
| Fanak et al., 2008 [23] | Iran | 64 | GI system disorders, Respiratory system disorders | - | Digoxin and Nitroglycerin |
| Gholami et al., 2008 [20] | Iran | 105 | CNS and GI system | Headache, vertigo | Diltiazem |
| Sharminder et al, 2009 [9] | India | 208 | CVS | Headache | Nitrates |
| Mohebbi et al., 2010 [24] | Iran | 189 | Nervous system disorders, GI system disorders | - | Nitroglycerin, Amiodarone |
| Iman et al., 2011 [25] | Iran | 70 | Nervous system disorders, GI system disorders | Headache and dizziness | Digoxin, Atenolol, and Streptokinase |
| Sharminder et al., 2011[10] | India | 208 | - | hypersensitivity skin reaction and headache | Nitrates and Diuretics |
| Singhal et al., 2011 [18] | India | 231 | CNS and GI system | Headache and dry cough | Calcium channel blockers and Nitrates |
| Arunkumar et al., 2013 [19] | India | 379 | Oral Cavity | Xerostomia, dysgeusia, burning sensation. | beta blockers, calcium channel blockers |
| Present study, 2013 | India | 463 | GI and Respiratory | Cough and gastritis | Enalapril and Aspirin |
GI-Gastro-intestinal system, CNS–Central Nervous system, CVS–Cardiovascular system
A study by Zaidenstein et al., found that, the causative drugs for ADRs were warfarin (25%), beta-blockers (15%), propafenone (5%), amiodarone (5%) and the most commonly observed ADRs were orthostatic hypotension, bleeding, arrhythmias etc [22]. Similarly another study by Fanak et al., carried out in post coronary care unit inpatients, found that the most common systems associated with ADRs were gastro-intestinal (14.1%) and respiratory system disorders (14.1%). Digoxin (14.1%) and nitroglycerin (14.1%) were the most commonly implicated drugs in causing ADRs [23]. The post coronary care unit inpatient settings of the above studies could be attributed to the contrary findings compared to that of the present study.
A study by Mohebbi et al., found that the most commonly affected system with ADR were central and peripheral nervous system disorders (23.5%) and gastro-intestinal system disorders (16.5%) with streptokinase (59.3%) and amiodarone (38.7%) as the drugs more frequently implicated with occurrence of ADR [24]. In the present study gastrointestinal system constituted 20.7% and respiratory system 18.4% of the ADR. A study by Iman et al., found that headache (15.7%) and dizziness (14.3 %) were the ADRs most frequently reported with central and peripheral nervous system disorders (37.1%) as well as gastrointestinal system disorders (21.4%) being most commonly involved systems. Digoxin, atenolol, and streptokinase were the most offending cardiovascular drugs of that study [25]. These variations in the occurrence of ADRs and drugs involved in causing frequent ADRs may be attributed to drug usage and prescription pattern of our hospital. However, present study has the advantage of reporting large number of ADRs observed with cardiovascular drugs in Indian population while all previous studies done were of limited duration with less sample size.
Conclusion
The present study found that gastrointestinal (20.7%) and respiratory systems (18.4%) as the most commonly affected organ systems owing to ADRs caused by cardiovascular drugs. The most frequently reported ADRs were dry cough and gastritis and the most commonly implicated cardiovascular drugs causing these ADRs were found to be enalapril, atorvastatin and aspirin. Since most patients with cardiovascular diseases are on multiple drugs it is not uncommon to see adverse drug reactions and it is important to monitor and alter therapy as and when the situation arises.
Others include Immune system disorders, Endocrine disorders, Eye disorders, Renal and urinary disorders, Blood and lymphatic system disorders, Ear and labyrinth disorders, Hepatobiliary disorders
ADR- adverse drug reaction CTCAE: Common Terminology Criteria for Adverse Events
ADR- adverse drug reaction
ADR- adverse drug reaction
GI-Gastro-intestinal system, CNS–Central Nervous system, CVS–Cardiovascular system