Laboratory Profiles of Patients on Hemodialysis - A Retrospective One Year Study in a Rural Tertiary Care Hospital
Udipi Badikillaya Vijayalakshmi1, Manasa Rayidi2
1 Associate Professor, Department of Biochemistry, Dr. Pinnamaneni Siddhartha Institute of Medical Sciences and Research Foundation (Dr. PSIMS & RF)Chinnavutapalli, Gannavaram Mandal, Krishna District, Andhra Pradesh, India.
2 Tutor, Department of Biochemistry, Dr. Pinnamaneni Siddhartha Institute of Medical Sciences and Research Foundation (Dr. PSIMS & RF)Chinnavutapalli, Gannavaram Mandal, Krishna District, Andhra Pradesh, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Udipi Badikillaya Vijayalakshmi, 40-14-4, Chandramoulipuram, Vijayawada-520010, Andhra Pradesh, India.
E-mail: drpvijayalakshmi68@gmail.com
Introduction
The global prevalence of chronic kidney disease (CKD) is estimated to be 8-16%. Studies have shown that the increased mortality in patients with CKD is due to anemia that leads to cardiovascular disease (CVD), also known as “Cardio renal anemia syndrome”. The present study was undertaken to look into the laboratory profiles of end stage renal disease (ESRD) patients.
Aim
To study the laboratory profiles of End stage renal disease (ESRD) patients coming for hemodialysis.
Materials and Methods
The study was a retrospective, cross- sectional study done by collecting data from the medical case records of all patients during a period of one year from January 1st 2014 to December 31st 2014. Records of a total of 140 patients who underwent hemodialysis during this period were taken. The laboratory profiles that was recorded included haemoglobin, serum sodium, potassium, chloride, fasting glucose, calcium and phosphorus.
Results
The mean age of the subjects was 53.5±14.5 yrs. All the patients had moderate anaemia. There was a significant difference in the mean systolic and diastolic blood pressure, serum creatinine and serum urea values between males and females. The mean serum calcium levels were low.
Conclusion
The present study is the first such study in this rural area and shows evidence of a relatively young population with ESRD having moderate anaemia and hypertension. There is evidence of hypocalcaemia and serum phosphorus is on the higher end of the normal range. These findings are usually associated with a higher risk of mortality. With the explosion of diabetes and hypertension in India, chronic kidney disease should be diagnosed and managed as early as possible if not prevented.
Anaemia, Chronic kidney disease, End stage renal disease, Hemodialysis
Introduction
Chronic kidney disease (CKD) is defined as either a reduced Glomerular filtration rate (GFR) of <60 ml/mt/1.73 m2 or albumin excretion or both over a period of three months. This is a major public health problem throughout the world [1]. The prevalence of CKD in the world according to a study by Jha et al., is around 8-16% [2]. In India the SEEK (screening and early evaluation of kidney disease) study has reported the age-adjusted incidence rate of ESRD (end stage renal disease) to be 229 per million population and it was found that >1,00,000 new patients enter renal replacement programs annually [3]. About 63,538 patients had enrolled in the Indian CKD registry. This registry is a voluntary reporting body of the patients suffering from CKD. Data indicate that out of the subjects enrolled 70% are males and a majority of them have stage 4 and 5 CKD and 20% of them are on renal replacement therapy [4]. A review by Thomas et al., has shown that the increased mortality in patients with CKD is due to anaemia that leads to cardiovascular disease (CVD), also known as “Cardio renal anaemia syndrome”[5]. The other cardiovascular risk factors associated with CKD are increased serum phosphorus level, abnormal calcium- phosphate ion product, parathormone levels and dyslipidemia [5]. Microalbuminuria is another cardiovascular risk factor in diabetic and hypertensive CKD [6]. The present study was undertaken to look into the laboratory profiles of ESRD patients over a period of one year. It was a retrospective study and the population studied was the patients coming for hemodialysis at a tertiary care hospital located in a rural area. The economically backward patients coming for dialysis are funded by a government scheme.
Aim
To study the laboratory profiles of all End stage renal disease (ESRD) patients coming for hemodialysis over a period of one year.
Materials and Methods
The study was a retrospective cross-sectional study done at Dr. PSIMS and RF a tertiary care teaching hospital in Chinnaavutapalli, in Krishna district - rural Andhra Pradesh. Data was collected from the medical case records over a period of one year from January 1st 2014 to December 31st 2014. Records of patients who underwent hemodialysis during this period were taken. The inclusion criterion was all subjects on hemodialysis and in stage 5 CKD. Those case records which did not have the relevant data or incomplete data were excluded. Paediatric and pregnant subjects were excluded. The patients included were in stage V CKD as assessed by clinicians based on eGFR (estimated Glomerular Filtration Rate) determined by Cockcroft-Gault equation and on maintenance hemodialysis (MHD). An eGFR value of <15 ml/mt/1.73m2 is considered stage V CKD [1]. A total of 140 patients met the inclusion criteria. The study was started after obtaining ethical committee clearance from the institute. The laboratory profiles included haemoglobin, serum sodium, potassium, chloride, fasting glucose, calcium and phosphorus. The blood pressure recorded in the case sheet and the clinical diagnosis was noted. The laboratory profiles recorded was after the last dialysis session, irrespective of the number of sessions the patients underwent. The treatment history, diet, drug history, duration and lifestyle factors were not taken into consideration.
Staristical Analysis
The results were recorded on Microsoft excel sheet and tabulated. Data are reported as mean and standard deviation. Students t-test was used to establish the differences between means of continuous variables and the results were considered significant when p <0.05.
Results
A total of 140 patients were included in the study. The demographic and laboratory profiles are given in [Table/Fig-1]. There was no significant age difference between the sexes. Out of 140 patients 20 were diabetics, 43 were hypertensive, 31 had a diagnosis of both diabetes and hypertension and 46 were neither diabetic nor hypertensive, but had other causes of CKD like analgesic nephropathy (10), infective cause of nephropathy (22) and for some the cause was not known (8). All the patients had moderate anaemia. There was a significant difference in the mean systolic and diastolic blood pressure between males and females, [Table/Fig-1]. There was a significant difference in serum creatinine and serum urea values between males and females (p<0.0001). There was no significant difference between the sexes in the other parameters. There were no significant differences in these parameters in those below 60 yrs of age and those above 60 years [Table/Fig-2]. The p-value was > 0.05. In all the patients mean serum calcium levels were low. The mean values of serum sodium were slightly lower in females when compared to males; [Table/Fig-1], though not statistically significant p value >0.05.
Gender differences in the clinico-demographic and laboratory parameters
| Parameters | Males (76)Mean ± SD | Females (64)Mean ± SD |
|---|
| Age (in yrs) | 55.2±14.8 | 52.6±14.2 |
| Hemoglobin (in g%) | 9.07±2.4 | 8.9±1.9 |
| Systolic blood pressure in (mmHg) | 162.5±13.7 | 179.6±30.4 (p=≤0.0001) |
| Diastolic blood pressure (in mmHg) | 89.8±17.0 | 84.2±12.4 (p=≤0.01) |
| Serum glucose (in mg/dl) (fasting) | 125.3±63.2 | 135.2±64.2 |
| Serum urea (in mg/dl) | 104.7±49.5 | 84.7±44.3 (p=≤0.0001) |
| Serum creatinine (in mg/dl) | 5.9±2.9 | 4.4±2.5 (p=≤0.0001) |
| Serum sodium (in meq/l) | 136.5±6.8 | 134.7±7.9 |
| Serum potassium (in meq/l) | 4.5±1.0 | 4.4±0.9 |
| Serum calcium (in mg/dl) | 8.2±0.8 | 8.2±0.6 |
| Serum phosphate (in mg/dl) | 3.9±0.5 | 4.1±0.3 |
Differences in the clinico-demographic and laboratory parameters in subjects ≥60 yrs of age and ≤60 yrs
| Parameters | age≥60 yrs | age≤ 60yrs |
|---|
| Number | 54 | 86 |
| Hemoglobin (in g%) | 9.0±2.5 | 8.9±1.9 |
| Systolic blood pressure (in mmHg) | 145.3±25.4 | 149.6±29.4 |
| Diastolic blood pressure (in mmHg) | 86.5±16.1 | 87.8±14.8 |
| Serum phosphorus (in mg/dl) | 3.9±0.4 | 4.0±0.4 |
| Serum calcium (in mg/dl) | 8.1±0.7 | 8.2±0.7 |
| Serum potassium (in meq/l) | 4.2±1.1 | 4.5±0.9 |
| Serum sodium (in meq/l) | 136.2±6.7 | 134.8±8.2 |
| Fasting serum glucose (in mg/dl) | 134.2±61.7 | 126.8±65.2 |
| Serum urea (in mg/dl) | 100.8±53.2 | 91.9±44.1 |
| Serum creatinine (in mg/dl) | 4.7±2.5 | 5.5±2.7 |
Discussion
The present study shows that the patients with end stage renal disease (ESRD) had a mean age of 53.5±14.5 yrs. The age distribution is depicted in [Table/Fig-3]. There are more subjects in the 51-60 yrs age group in this study. In countries like Nigeria there are more subjects in the 41-50 yr age group [7]. The elderly were the ones affected with CKD in developed countries probably because of improved life expectancy [2]. More males were affected than females in this study. Females were on an average 2 years younger than males (52.6 years vs. 55.2 years). These values are similar to a study done by Rajapurkar et al., which reported the mean age as 50.1±14.6 yrs and males were older to females (50.9 ±14.6 vs. 48.3 ± 14.4 years) [8]. More males were affected according to the screening and early evaluation of kidney disease (SEEK) study in India [3]. The SEEK study reported 55.1% males and 44.9% females which is similar to our study where males were 54.3% and females were 45.7% . The SEEK study also showed that the incidence of CKD was highest in Visakhapatnam, Andhra Pradesh (48%) compared to Bangalore (4%) [3].
Age distribution of the subjects
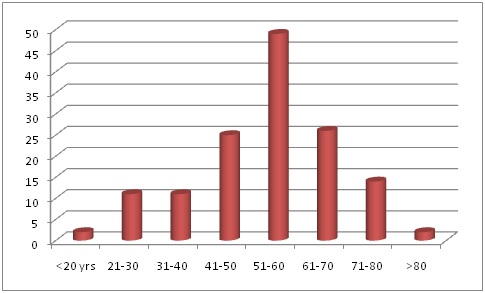
The most common cause of CKD in the developing and developed countries was diabetes mellitus (DM) [2]. The present study shows that 43 subjects out of 140 were hypertensive and 20 were subjects with only DM and 31 had a diagnosis of both DM and hypertension. The SEEK study also reported a higher incidence of hypertension in CKD patients when compared to DM (64.5% had hypertension and 31.6% had DM) [3]. Our findings are in consonance with the SEEK study and the number of patients with hypertension is higher than DM. Both high systolic and diastolic blood pressure have been linked to the development of ESRD. A study by Tozawa et al., has reported that high blood pressure is an independent risk factor for both diabetic and non diabetic ESRD [9].
The mean serum urea and creatinine values were high; being 95.6±48 mg/dl (normal-15-40 mg/dl) and 5.2±2.8 mg/dl (normal-0.5-1.5mg/dl) respectively and the difference in these values between males and females was statistically significant, the values being higher in males than in females [Table/Fig-1]. These values are indicative of CKD [1]. All the subjects had moderate anaemia (anaemia classified based on WHO classification), with a mean haemoglobin (Hb) concentration being around 9.0±2 g/dl [10]. The KDOQI-NKF 2012 guidelines suggest that Hb levels should be corrected to 11 g/dl or above to prevent secondary complications due to anaemia [11]. The complications that arise due to anaemia are left ventricular hypertrophy (LVH), cardiac failure, exercise intolerance and defective cognitive functions [5,12]. It has been reported in a study that for every 1 g/dl fall in haemoglobin, the mortality rises by 18-25% and the risk of LVH increases by 50% [13]. Another study reported that an increase in mean Hb of 2.7 g/dl was accompanied by a decreased left ventricular mass index in almost all the patients. This occurs even in the absence of blood pressure control [14]. The cause of anaemia in CKD is multi factorial. Early studies on CKD found decreased erythropoietin as a cause of anemia. Later several factors were identified that included, haemolysis due to uremic toxins, disordered iron homeostasis due to increased hepcidin (a protein- regulating iron levels), frequent phlebotomies and trapping of blood in the dialysis apparatus [12].
The mean serum calcium levels were 8.1±0.7 mg/dl (normal value 9-11 mg/dl) and mean serum phosphorus levels were 3.9±0.4mg/dl (normal value-2.5-4 mg/dl). A study by Miller et al., showed that a low serum calcium <9 mg/dl along with a high serum phosphorus of >3.5 mg/dl was associated with greater mortality [15]. Hypocalcaemia occurs as a consequence of hyperphosphatemia and decreased calcitriol. A disordered kidney in ESRD is incapable of excreting phosphate and synthesizing calcitriol. Hyperphosphatemia leads to stimulation of parathyroid gland causing parathormone (PTH) release. It also increases the production of fibroblast growth factor 23 (FGF 23 secreted mainly by osteocytes), which has an inhibitory effect on 1-α hydroxylase enzyme. This leads to decreased calcitriol and decreased absorption of calcium from the intestine. The increased phosphate causes hypocalcemia by precipitating with calcium and forming calcium hydroxyapatite (CaHPO4) crystals. The down-regulation of calcitriol receptors on the parathyroid gland leads to vitamin D resistance. The loss of negative feedback on the parathyroid gland causes increased PTH. Parathormone stimulation causes increased osteoclastic activity, which in turn leads to increased calcium phosphate product and precipitation of CaHPO4 extra skeletally. This further reduces serum calcium. The secondary hyperparathyroidism so produced leads to renal osteodystrophy [16]. Hypocalcaemia states can precipitate adverse cardiac outcomes like cardiomyopathy, congestive cardiac failure, ventricular tachycardia and other arrhythmias [15]. These arrhythmias may lead to sudden death which is a common cause of mortality in haemodialysis patients. The KDOQI guidelines recommend a target value for serum calcium between 8.4 to 9.5 mg/dl. A study by Kestenbaum et al., has revealed an association between high serum phosphorus level and increased risk of mortality. Higher serum phosphorus levels were also associated with female gender, DM, lower GFR and low Hb [17]. They also found a linear relationship between serum phosphate level and mortality for each 0.5 mg/dl rise in serum phosphate, with the highest mortality being observed with values above 3.5 mg/dl [16]. Our study did not show any relation between high serum phosphorus and female gender, DM or Hb. Kovesdy et al., also showed an increased risk of mortality with a serum phosphate value of >4 mg/dl [18]. Abnormal mineral metabolism occurs early in the course of CKD and ESRD. Hyperphosphatemia is associated with a greater cardiovascular morbidity and mortality [18].
The mean serum sodium and potassium levels were 135.7±7.1 meq/l and 4.4±0.9 meq/l respectively. The mean values of serum sodium were slightly lower in females when compared to males being 134.7±7.9 versus 136.5±6.8 meq/l, but there was no significant difference. A study by Mandai et al., has reported that low serum sodium is an independent predictor of a higher risk of infection in maintenance hemodialysis patients [19]. The mean serum sodium levels in females were not very low being 134.7meq/l (normal range 135-145 meq/l). Korgaonkar et al., in a study have reported that low or low normal serum potassium levels of 3.5-4.0 meq/l are associated with a greater risk of mortality [20]. The patients in the present study had a mean serum potassium value of 4.4 meq/l (normal range 3.5-5 meq/l). The only way to reduce the risk of mortality in CKD patients is regular assessment of laboratory parameters as has been suggested in KDOQI guidelines on CKD and correcting abnormalities as indicated. Anaemia should be assessed depending on clinical indicators in patients with GFR >60 ml/mt/1.73m2, yearly in patients with GFR between 30-59 ml/mt/1.73 m2 and twice a year in those with GFR <30 ml/mt/1.73 m2 [1]. Patients should be given either erythropoietin stimulating agents (ESA) or iron therapy depending on the staging of CKD and whether they are on maintenance hemodialysis or not [21]. It is recommended to monitor serum calcium, phosphorus, alkaline phosphatase and PTH at least yearly in patients with GFR <45 ml/mt/1.73m2. When intact serum PTH levels are above the normal range in this group of patients, they should be monitored for hyperphosphatemia, hypocalcaemia and vitamin D deficiency. Vitamin D should be prescribed in those patients having a documented deficiency [1]. Dietary restriction of phosphorus is advised and use of phosphate binders as indicated to reduce hyperphosphatemia [22]. Calcium supplements are not advised in stages 3 and 4 of CKD and even in stage 5 it should be given judiciously as the risk of coronary calcification should be kept in mind [23]. All these precautions only help in delaying cardiac complications. Dysnatremias have to be detected by regular laboratory investigations as indicated [1]. Hyperkalemia in dialysis patients can be prevented by dietary potassium restriction to 1.5-2 g/day [24].
Limitations
The inherent limitations of the study are a lack of follow up of these subjects to study the effects of anaemia, hypocalcaemia and increased serum phosphorus on mortality. As this was a descriptive study, the effects of the number of dialysis sessions, duration of CKD, drugs used and diet taken were not recorded. These also have a bearing on the laboratory values. The role of the above mentioned factors will be addressed during the follow up of these patients. We also plan to study the effect of improving anaemia on mortality.
Conclusion
The present study is the first such study in this rural area and shows evidence of moderate anaemia, low calcium and increased phosphorus levels in these subjects. These subjects are relatively younger and hypertensive as in the SEEK study. As the number of subjects on hemodialysis is high we plan to follow up these patients, to study the causes of mortality and the risk of adverse cardiovascular outcomes. It can be seen from this study that diabetes and hypertension are the primary causes of CKD. A change in the food habits and lifestyle of Indians is responsible for the high prevalence of lifestyle disorders like DM and hypertension leading to CKD. There should be increased awareness of this common complication of these lifestyle disorders so that we can aim at prevention or early intervention in CKD.
[1]. Inker LA, Astor BC, Fox CH, Isakova T, Lash JP, Peralta CA, KDOQI US Commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKDAm J Kidney Dis 2014 63(5):713-35. [Google Scholar]
[2]. Jha V, Garcia GG, Isek K, Li Z, Naicker S, Plattner B, Chronic kidney disease: global dimension and perspectives. [internet] 2013 [cited 2013 May 31] Available from www.thelancet.com.http://dx.doi.org/10.1016/S0140-6736(13)60687-X [Google Scholar]
[3]. Singh AK, Farag YMK, Mittal BV, Subramanian KK, Reddy SRK, Acharya VN, Epidemiology and risk factors of chronic kidney disease in India – results from the SEEK (Screening and Early Evaluation of Kidney Disease) studyBMC Nephrol 2013 14:114doi:10.1186/1471-2369-14-114 [Google Scholar]
[4]. Veerappan I, Abraham G, Chronic kidney disease: current status, challenges and management in India. [internet] 2008 Available from www.apiindia.org/pdf/pg_med_2008/Chapter-44.pdf [Google Scholar]
[5]. Thomas R, Kanso A, Sedor JR, Chronic kidney disease and its complicationsPrim Care Clin Office Pract 2008 35(2):329-44. [Google Scholar]
[6]. Basi S, Mimran A, Fesler P, Lewis JB, Microalbuminuria in type 2 diabetes and hypertensionDiabetes Care 2008 31:S2-194-201.doi:10.2337/dc08-s249 [Google Scholar]
[7]. Amira CO, Bello BT, Braimo RW, Chronic kidney disease: a ten-year study of aetiology and epidemiological trends in Lagos, NigeriaBr J Ren Med 2014 19(4):19-22. [Google Scholar]
[8]. Rajapurkar MM, John GT, Kirpalani AL, Abraham G, Agarwal SK, Almeida AF, What do we know about chronic kidney disease in India: first report of the Indian CKD registryBMC Nephrol 2012 13:10doi:10.1186/1471-2369-13-10 [Google Scholar]
[9]. Tozawa M, Iseki K, Iseki C, Kinjo K, Ikemiya Y, Takishita S, Blood pressure predicts risk of developing end stage renal disease in men and womenHypertension 2003 41(6):1341-45. [Google Scholar]
[10]. WHO. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Vitamin and Mineral Nutrition Information System. Geneva, World Health Organization 2011(WHO/NMH/NHD/MNM/11.1). [internet] available at (http://www.who.int/vmnis/indicators/haemoglobin.pdf.) [Google Scholar]
[11]. Kidney disease: improving global outcomes (KDIGO) anemia work group. KDIGO clinical practice guideline for anemia in chronic kidney diseaseKidney Int Suppl 2012 2(4)(S(2)):279-335. [Google Scholar]
[12]. Babitt JL, Lin HY, Mechanisms of anemia in CKDJ Am Soc Nephrol 2012 23(10):1631-34. [Google Scholar]
[13]. Datta S, Abraham G, Mathew M, Somasundaram H, Muralidharan TR, Asha Moorthy, Correlation of anemia, secondary hyperparathyroidism with left ventricular hypertrophy in chronic kidney disease patientsJ Assoc Physicians India 2006 54(9):699-703. [Google Scholar]
[14]. Levin A, Thompson CR, Ethier Carlisle EJ, Toby S, Mendelsson D, Left ventricular mass index increase in early renal disease: impact of decline in hemoglobinAm J Kidney Dis 1999 34(1):125-34. [Google Scholar]
[15]. Miller JE, Kovesdy CP, Norris KC, Mehrotra R, Nissenson AR, Kopple JD, Association of cumulatively low or high serum calcium levels with mortality in long-term hemodialysis patientsAm J Nephrol 2010 32(5):403-13.doi: 10.1159/000319861 [Google Scholar]
[16]. Saliba W, El-Haddad B, Secondary hyperparathyroidism: pathophysiology and treatmentJ Am Board Fam Med 2009 22(5):574-81. [Google Scholar]
[17]. Kestenbaum B, Sampson JN, Rudser KD, Patterson DJ, Seliger SL, Young B, Serum phosphate levels and mortality risk among people with chronic kidney diseaseJ Am Soc Nephrol 2005 16(2):520-28. [Google Scholar]
[18]. Kovesdy CP, Ahmadzadeh S, Anderson JE, Kalantar-Zadeh K, Secondary hyperparathyroidism is associated with higher mortality in men with moderate to severe chronic kidney diseaseKidney Int 2008 73(11):1296-1302.doi:10.1038/ki.2008.64 [Google Scholar]
[19]. Mandai S, Kuwahara M, Yuri Kasagi Y, Kusaka K, Tanaka T, Shikuma S, Lower serum sodium level predicts higher risk of infection-related hospitalization in maintenance hemodialysis patients: an observational cohort studyBMC Nephrol 2013 14:276doi:10.1186/1471-2369-14-276 [Google Scholar]
[20]. Korgaonkar S, Tilea A, Gillespie BW, Kiser M, Eisele G, Finkelstein F, Serum potassium and outcomes in CKD: insights from the RRI-CKD Cohort StudyClin J Am Soc Nephrol 2010 5(5):762-69.doi: 10.2215/CJN.05850809 [Google Scholar]
[21]. O’Mara NB, Anemia in patients with chronic kidney diseaseDiabetes Spectr 2008 21(1):12-19.doi:10.2337/diaspect.21.1.12 [Google Scholar]
[22]. Ketteler M, Phosphate metabolism in CKD stages 3-5: dietary and pharmacological controlInt J nephrol 2011 Article ID 970245 doi:10.406/2011/970245 [Google Scholar]
[23]. Langman CB, Cannata–Andia JB, Calcium in chronic kidney disease: myths and realitiesClin J Am Soc Nephrol 2010 5:S1-2.doi: 10.2215/CJN.06140809 [Google Scholar]
[24]. Allon M, Hyperkalemia in end stage renal disease: mechanisms and managementJ Am Soc Nephrol 1995 6:1134-42. [Google Scholar]