Candiduria in Catheter Associated Urinary Tract Infection with Special Reference to Biofilm Production
Mythreyi Shekar Rishpana1, Jyoti S Kabbin2
1 Student, Bangalore Medical College and Research Institute, Bangalore, India.
2 Professor, Department of Microbiology, Bangalore Medical College and Research Institute, Bangalore, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Jyoti S Kabbin, H.No.701, A wing, Brigade Paramount, Opp. to RMZ Infinity, Old Madras Road, CV Raman Nagar, KR Puram, Bangalore-560093, India.
E-mail: drjyotiskabbin@gmail.com
Introduction
Urinary tract infections as a result of Candida species are becoming increasingly common in hospital settings. The association is higher in patients with prolonged urinary catheterization and also various pre-disposing factors.
Aim
This study was done to look into the significance of candiduria in the catheterized patients and to perform microbial catheterization of yeast and biofilm detection by tube method to guide treatment protocol.
Materials and Methods
This is a prospective study. One hundred urine samples were collected over a period of 3 months. Specimens included were those of patients presenting with nosocomial Urinary tract infection (UTI) after 72 hours of hospitalization. The urine samples obtained were immediately processed in microbiology laboratory by semi-quantitative method as per standard protocol. All yeast isolates were stored for further microbial characterization. Biofilm production was detected by tube method.
Results
In the present study we observed that out of 100 samples obtained from catheterized patients presenting with nosocomial UTI 26% were caused by Candida species. Among the 26 Candida isolates 16 (61.53%) were non albicans Candida and 10(38.47%) were Candida albicans. Out 26 Candida isolates, 14(53.84%) of the candida isolates were found to produce biofilm. Biofilm production was found to occur more frequently among non albicans Candida 10(62.5%) than Candida albicans 4(40.0%).
Conclusion
The present study reiterates the presence of candiduria in catheterized patients. Non-albicans candida speices are replacing candida albicans as the predominant pathogen for nosocomial UTI. It was also observed that Biofilm formation is seen more frequently with non albicans candida species than with Candida albicans.
Candida spp., Catheterization, Nosocomial Urinary tract infection
Introduction
Nosocomial infections are applied to infections developing in hospitalized patients, not present or without incubation at the time of their admission. These infections are frequently opportunistic and are associated with microorganisms of low virulence and patients with impaired immunity. The source of hospital acquired infections is usually exogenous in nature and may be derived from any part of the ecosystem in the hospital. Such nosocomial infections add to the morbidity, mortality, and costs that one might expect from the underlying illness alone. This is tragic since it is believed that as many as 40 per cent of nosocomial infections in developing countries are preventable [1].
Candiduria is seldom encountered in healthy individuals. The prevalence of candiduria is higher among hospitalized patients with indwelling devices and accounts for around 10 to 15% of nosocomial urinary tract infections (UTIs) [2–4].
The prevalence of true infection has increased significantly over the past few years due to the presence of various predisposing factors in hospitalized patients [5]. The predisposing factors frequently associated with candiduria are urinary tract instrumentation, prior antibiotic use, prolonged hospitalized stay, extremes of age, diabetes mellitus, female sex and use of immunosuppressive therapy [6]. An aggressive approach is vital in management of Candidemic patients with underlying risk factors for prevention and early diagnosis of disseminated candidiasis [7].
Candida albicans is the most common yeast isolated in patients with UTI. However, there are reports of changing pattern with a rising prevalence of non-albicans candida. The inherent resistance of non-albicans Candida to fluconazole is well documented, necessitating speciation of Candida in patients with UTI for initiation of appropriate therapy [8].
The significance of indwelling devices in hospitalized patients lies in the fact that these are very frequently associated with formation of biofilms on mucosal surface and plastic surface of indwelling devices, which consist of a complex enclosing micro-colonies of yeast, hyphae, and pseudo hyphae. These biofilms are inherently resistant to anti-fungal agents including amphotericin B and fluconazole, rendering them ineffective during the treatment of candiduria. Affected devices need to be removed to ensure proper management. The biofilm producing capacity varies among the species of Candida [9].
Materials and Methods
After obtaining clearance from Institutional Ethics Committee (IEC), this study was done in the Department of Microbiology of Bangalore Medical College and Research Institute (BMCRI), a tertiary care teaching hospital in Bengaluru, Karnataka. The present study was carried out over a period of three months during the period between May and July 2013. Hundred urine samples were collected aseptically using the standard protocol from catheterized patients admitted in a tertiary care hospital attached to BMCRI. Informed consent was collected from all the subjects included under the study. These specimens were transported immediately to the Department of Microbiology for processing. Specimens included were those of patients presenting with suspected nosocomial UTI after 72 hours of hospitalization. Hospitalized patients without urinary catheterization or absence of pyuria, duration of stay in the hospital being less than 72 hours and mixed growth in culture were exclusion criteria for the present study [5].
Urine sample processing and identification
The urine samples obtained were immediately processed in microbiology laboratory by semi-quantitative method as per standard protocol [5]. All yeast isolates were stored for further microbial characterization. Direct microscopic examination is also done to look for pus cells, blood cells, casts, crystals or any bacterial or fungal elements.
All yeast samples that grew on Sabouraud dextrose agar medium (SDA) after gram staining were sub cultured on chromogenic medium (Hi CHROMagar, Hi Media, Mumbai, India), which was used as a follow up media. Yeast isolates sub cultured on chromogenic agar were incubated overnight at 350C. The plate were further incubated for a total incubation of 48 hours to get better developed coloured colonies. Presumptive identification was made by colour and morphology of the colonies as per manufacturer’s instructions. These isolates were further identified on the basis of microscopic morphological features of the growth obtained from Sabouraud dextrose agar medium (SDA) culture [10].
Biofilm formation
Biofilm production was detected by tube method described by Brachini et al., loopful of organisms from SDA were inoculated into Sabouraud’s Dextrose broth supplemented with glucose (final concentration 8%). The tubes were incubated at 370C for 24 hours after which broth is aspirated out gently. The tubes were then washed once with distilled water and then stained with 1% Saffranin. The tubes are then kept still for 7 minutes. Saffranin is removed and tubes are examined for biofilm production. Biofilm production is tested twice and read independently by two different observers. The adherent biofilm layer is scored visually as negative or positive (1+), moderate positive (2+) or strong positive (3+) [11].
Results
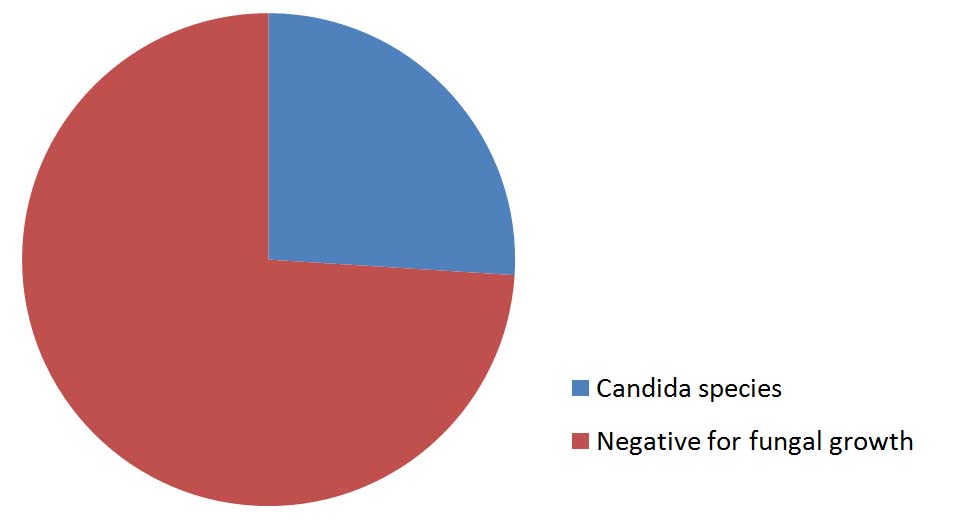
During the study period, laboratory data of 100 patients whose specimens were received was evaluated. Male to female ratio was 2.3:1. Specimens were those of patients catheterized for more than 72 hours presenting with nosocomial UTI. Out of 100 urine sample specimens tested 26 (26%) were positive for candida species. The remaining 74(74%) did show fungal growth [Table/Fig-1,2].
Candida species isolated from urine sample
| Number of Isolates | Percentage (%) |
|---|
| Candida species | 26 | 26 |
| Negative for fungal growth | 74 | 74 |
Candida species isolated from urine sample
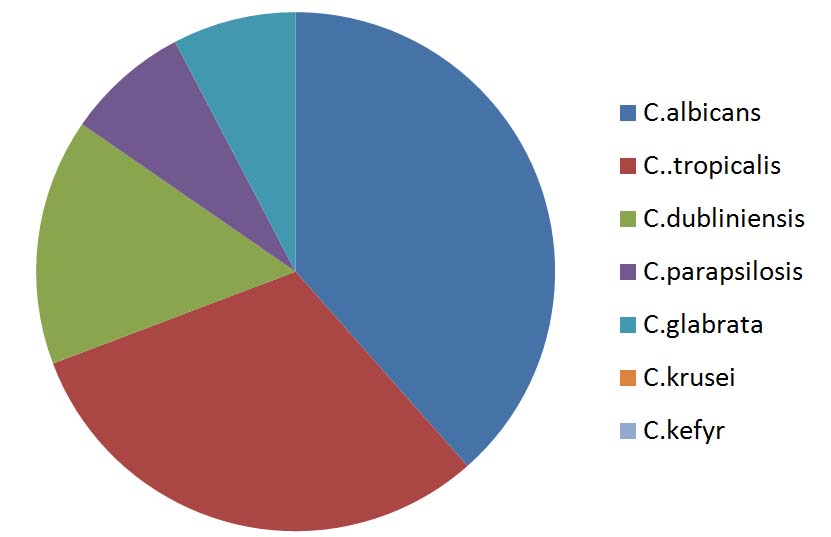
Among the 26 Candida isolate, 16(61.53%) were Non albicans Candida species and 10(38.47%) were Candida albicans. Among the non-Candida albicans the most common isolate was C.tropicalis 8(30.76%) followed by C.dublisiense 4(15.38%). Others were C.glabarta, C.parapsilosis, C.krusei and C.kefyr [Table/Fig-3,4,5,6 and 7].
Candida species isolated from urine sample of catheterized patients
| Candida Species | Number of Isolates | Percentage (%) |
|---|
| C.albicans | 10 | 38.47 |
| C..tropicalis | 8 | 30.76 |
| C.dubliniensis | 4 | 15.38 |
| C.parapsilosis | 2 | 7.69 |
| C.glabrata | 2 | 7.69 |
| C.krusei | 0 | 0 |
| C.kefyr | 0 | 0 |
| Total | 26 | 100 |
Candida species isolated from urine sample of catheterzied patients
Biofilm production by different candida species
| CandidaSpecies | Numberof Isolates | Numberof BiofilmPositiveIsolates | BiofilmGrading | Perce-ntageof Biofilm +VeIsolate (%) |
|---|
| | | 3+ | 2+ | 1+ | |
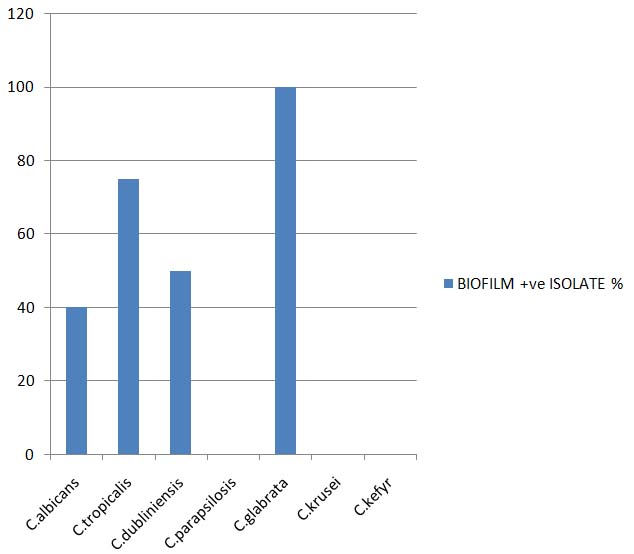
| C.albicans | 10 | 4 | 0 | 2 | 2 | 40 |
| C.tropicalis | 8 | 6 | 2 | 4 | 0 | 75 |
| C.dubliniensis | 4 | 2 | 0 | 0 | 2 | 50 |
| C.parapsilosis | 2 | 0 | 0 | 0 | 0 | 0 |
| C.glabrata | 2 | 2 | 0 | 2 | 0 | 100 |
| C.krusei | 0 | 0 | 0 | 0 | 0 | 0 |
| C.kefyr | 0 | 0 | 0 | 0 | 0 | 0 |
Growth of Candida species on CHROMagar


Out of the 26 Candida isolates 14(63.63%) were found to be biofilm producers. Biofilm production was found to occur more frequently among non albicans Candida 10 (62.5%) than Candida albicans 4(40.0%). The prevalence of Biofilm production among non albicans Candida species is depicted in the [Tables/Fig-8].
Biofilm production by different candida species
Discussion
Nosocomial UTI is the most common health care associated infection [12]. In the present study, we observed that out of 100 urine samples obtained from catheterized patients presenting with nosocomial UTI. 26% were caused by Candida species [Table/Fig-1]. Among the 26 Candida isolates 16 (61.53%) were non albicans Candida and 10 (38.47%) were Candida albicans. Our data indicates a trend towards an increasing prevalence of infections caused by species of non albicans Candida.
Among non albicans Candida species, C.tropicalis 8(30.76%) was most commonly encountered, followed by C.dubliensis 4(15.38%), C.parapsilosis 2(7.69%) and C.glabrata 2(7.69%) [Table/Fig-2,3 and 4]. The pattern of non albicans Candida is in concordance with studies conducted by Jain M et al., and Golia S et al., however, the findings of the present study shows a rise in the prevalence of non albicans Candida as compared to the study by Golia S et al., [5,13] [Table/Fig-4,6].
In the present study, 14(53.84%) of the Candida isolates were found to produce biofilm. Biofilm production was found to occur more frequently among non albicans Candida 10 (62.5%) and Candida albicans 4(40.0%). This is in concordance with the study conducted by Golia S et al., [13] [Table/Fig-9,10].
Comparison of data obtained from present study with that of established literatures
| Candida Species | Study by Jain M et al.,Percentage (%) | Present StudyPercentage (%) |
|---|
| C.albicans | 28.6 | 38.47 |
| C.tropicalis | 52.9 | 30.76 |
| Othernon- species | 18.5 | 30.77 |
Comparison of Biofilm production among candida species isolated
| Number ofisolatestestedfor Biofilmproduction | PercentageofC.albicans-speciesisolated | Percentageof nonalbicansCandidaSpeciesisolated | Percentageof biofilmproducers | Percentageof C.albicansspeciesproducingbiofilm | Percentageof nonalbicansCandida Speciesproducingbiofilm |
|---|
| Study by S Goliaet al., [13](2012) | 108 | 43.37% | 54.63% | 65.74 % | 38.03% | 38.03% |
| Present study | 26 | 38.47% | 61.53% | 63.63% | 40.0% | 62.5% |
Limitations
The sample size in the present study is small and the findings have to be confirmed with a larger sample size. Another limitation of the study is that antifungal susceptibility was not carried out. As non-albicans Candida are more difficult to treat the chances that these strains would remain persistent are higher.
Conclusion
This study documents the prevalence of candiduria in catheterized patients and the change in trend with shift toward non albicans Candida species as the predominant pathogen causing nosocomial UTI. Biofilm formation is seen more frequently with non albicans candida species than with Candida albicans and as biofilm production may help maintain the role of fungi as commensals and pathogen, by evading host defense mechanisms, resisting fungal treatment and withstanding the competitive pressure from other organisms, these are difficult to treat. Hence, we conclude that species identification and biofilm detection must be performed for early and effective treatment of the patient. Biofilm formation by Candida species (by 63.63% of the isolates) confers additional resistance to treatment and hence there is an indication for rapid diagnosis and management of such patients.
[1]. Urinary Tract Infections, In: Kapil A, EditorAnanthanarayan and Paniker’s Textbook of Microbiology 2013 9th edHyderabadUniversities Press (India) Private Limited [Google Scholar]
[2]. Bukhary ZA, Candiduria: A review of clinical significance and managementSaudi J Kidney Dis Transpl 2008 19(3):350-60. [Google Scholar]
[3]. Kauffman CA, CandiduriaClin Infect Dis 2005 41(Suppl 6):S371-76. [Google Scholar]
[4]. Schonebeck J, Ansehn S, The occurence of yeast-like fungi in the urine under normal conditions and in various types of urinary pathologyScand J Urol Nephrol 1972 6(2):123-28. [Google Scholar]
[5]. Jain M, Dogra V, Mishra B, Thakur A, Loomba PS, Bhargava A, Candiduria in catheterized intensive care unit patients : emerging microbiological trendsIndian J Pathol Microbiol 2011 54(3):552-55. [Google Scholar]
[6]. Kauffman CA, Vazquez JA, Sobel JD, Gallis HA, McKinsey DS, Karchmer AW, Prospective multicenter surveillance study of funguria in hospitalized patientsClin Infect Dis 2003 30(1):14-18. [Google Scholar]
[7]. Toya SP, Schraufnagel DE, Tzeleps GE, Candiduria in intensive care units: association with heavy colonization and candedemiaJ Hosp Infect 2007 66(3):201-06. [Google Scholar]
[8]. Gubbins PO, Piscitelli SC, Danziger LU, Candida urinary tract infections: a comprehensive review of their diagnosis and managementPharmacotherapy 1993 13:110-27. [Google Scholar]
[9]. Tumbarello M, Posteraro B, Trecarichi EM, Fiori B, Rossi M, Biofilm production by Candida species and inadequate antifungal therapy as predictors of mortality for patients with candidemiaJ. Clin. Microbiol 2007 45(6):1843-50. [Google Scholar]
[10]. Agarwal S, Manchand V, Verma N, Yeast identification in routine laboratoryIndian J Med Microbiol 2011 29(2):172-77. [Google Scholar]
[11]. Branchini ML, Pfaller MA, Rhine-Chalberg J, Frempong T, Isenberg H.D, Genotypic variation and slime production among blood and catheter isolatesJ ClinMicrobiol 1994 Feb 32(2):452-456. [Google Scholar]
[12]. Fisher JF, Newman CL, Sobel JD, Yeast in the urine: Solution for a budding problemClin Infect Dis 1995 20(1):183-89. [Google Scholar]
[13]. Golia S, Hittinahalli V, Sangeetha K.T, Vasudha C.L, Study of Biofilm formation as a virulence marker in candida species isolated from various clinical specimensJEMDS 2012 1(6):1238-45. [Google Scholar]