Acute Hypercalcaemia and Hypervitaminosis D in an Infant with Extra Pulmonary Tuberculosis
Devi Dayal1, Siya Ram Didel2, Sikha Agarwal3, Naresh Sachdeva4, Meenu Singh5
1 Additional Professor, Pediatric Endocrinology & Diabetes Unit, Department of Pediatrics, Postgraduate Institute of Medical Education and Research, Chandigarh, India.
2 Registrar, Department of Pediatrics, Postgraduate Institute of Medical Education and Research, Chandigarh, India.
3 Registrar, Department of Pediatrics, Postgraduate Institute of Medical Education and Research, Chandigarh, India.
4 Assistant Professor, Department of Endocrinology, Postgraduate Institute of Medical Education and Research, Chandigarh, India.
5 Professor, Department of Pediatrics, Postgraduate Institute of Medical Education and Research, Chandigarh, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Devi Dayal, Additional Professor, Pediatric Endocrinology & Diabetes Unit, Department of Pediatrics, Advanced Pediatrics Center, Postgraduate Institute of Medical Education and Research, Chandigarh-160012, India. E-mail : drdevidayal@gmail.com, dayal.devi@pgimer.edu.in
In patients with tuberculosis, abnormal extrarenal production of 1,25-dihydroxyvitamin D3 by activated macrophages in granulomatous tissues may result in hypercalcaemia. More commonly reported in adults with active pulmonary tuberculosis, this complication may rarely occur in extrapulmonary tuberculosis, and children. The hypercalcaemia may be precipitated by usually recommended vitamin D and calcium supplementation in patients with tuberculosis. We report here an infant with tubercular meningitis who developed hypercalcaemia 12 days after starting routine vitamin D and calcium supplementation. This communication highlights the importance of close monitoring of calcium levels in patients with tuberculosis, especially if started on vitamin D and calcium replacement before anti-tubercular therapy.
Hypercalcaemic crisis, Vitamin D toxicity, Tubercular meningitis
Case Report
A 10-month-old girl presented with moderate grade fever for 3 weeks, one episode of seizure on day 3 of illness followed by weakness of right upper and lower limbs and alteration of sensorium for 2 weeks. There were no other systemic complaints except decreased feeding and lethargy. There was no history of contact with TB. She had received ceftriaxone and vancomycin for 10 days prior to presentation as her cerebrospinal fluid (CSF) analysis showed high protein and cell count, and low glucose but no microorganisms. She was referred to us due to persistence of fever. On examination, her weight and length were 6.4 kg and 70.0 cm respectively (-2.0 and -0.2 SDS respectively on WHO Growth Charts 2006). Neurological examination revealed decreased power (3/5) in right upper and lower limbs, brisk deep tendon reflexes and positive signs of meningeal irritation. Rest of her systemic examination was unremarkable. A provisional diagnosis of chronic meningitis was considered. Awaiting reports of planned investigations, child was continued on ceftriaxone and vancomycin, and also started on routine vitamin D (400 IU/day) and calcium (25 mg/kg/day) supplementation.
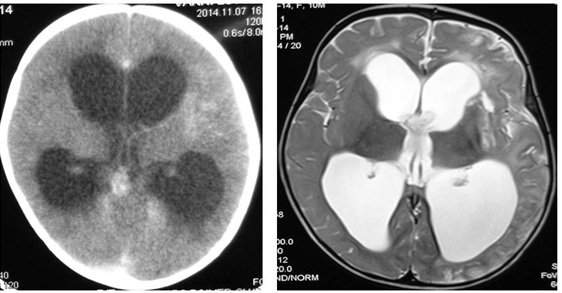
Investigations showed haemoglobin of 9.2 g/dL (normal, 11.5-15.5 g/dL), leukocyte count 7100/mm3 (normal, 5500-15,500/mm3) and platelets 4.84×103/mm3 (normal, 1.5-4.0×103/mm3). Liver and renal function tests were normal. The C-reactive protein was 8.2 mg/L (normal <5 mg/L) and erythrocyte sedimentation rate 15 mm/hour. All microbiological investigations (blood cultures, urine microscopy and culture) were normal. Chest radiograph and ultrasonogram of abdomen also showed no abnormality. Cranial sonography showed Ventriculo-Hemispheric Ratio (VHR) of 0.57 (normal 0.30±0.02). Contrast enhanced CT and Magnetic Resonance Imaging (MRI) showed extraventricular obstructive hydrocephalus, and gliosis involving cerebral cortex, white matter and basal ganglia on left side [Table/Fig-1]. The CSF analysis showed leukocyte count of 160/μL (normal 0-8 lymphocytes) with 80% lymphocytes, protein 98 mg/dL (normal 15-45 mg/dL), glucose 15 mg/dL (normal 40-80 mg/dL), and adenosine deaminase (ADA) level 45 U/L (normal level <10 U/L). Gram and acid-fast bacillus (AFB) stain, TB-PCR as well as culture of CSF were negative. Examination of CSF for other organisms such as Toxoplasma gondii, Cryptococcus and fungi was negative. Mycobacterial staining of gastric aspirate was negative. The Mantoux tuberculin test showed an induration of 10 mm (normal <5mm). Anti-tubercular therapy (ATT) was initiated after 2 weeks of hospital stay.
Contrast enhanced CT and MRI scan of the patient showing extraventricular obstructive hydrocephalus and features of meningovasculitis
Child developed vomiting, poor feeding and lethargy on day 12 of hospitalisation. This was initially attributed to worsening of hydrocephalus, but the repeat VHR was 0.55. Routine biochemistry showed hypercalcaemia. Serum albumin and blood pH were normal. Investigations were sent for etiology and effects of hypercalcaemia and are shown in [Table/Fig-2]. The vitamin D and calcium supplementation was stopped and the child was initiated on intravenous hyperhydration. Intravenous furosemide (2 mg/kg/dose, 6 hourly) and oral potassium (2 mEq/kg/day) were added the next day. Hypercalcaemia worsened despite forced diuresis for 2 days. Child was then given two subcutaneous doses of calcitonin (4 IU/kg) 12 hours apart. Ionised calcium decreased from 1.77 to 1.22 mmol/L and total calcium from 14.5 to 12.2 mg/dL. No further doses of calcitonin were given but hyperhydration was continued for another 3 days. There was neither a recurrence of hypercalcaemia nor the related symptoms over the next 2 weeks of hospitalisation. She has remained asymptomatic over 5 months of follow up and continues on ATT. An ultrasonogram done during last follow up showed no nephrocalcinosis. A written informed consent from parents was obtained for using the patient details and clinical images.
Biochemical data of the patient before and after the episode of hypercalcemia
| Hospitalday | Serumcalcium(mg/dL) | Ionisedcalcium(mmol/L) | Serumphosphate(mg/dL) | Serum 25(OH)2D3(ng/mL) | Serum 1,25(OH)2D3(pg/mL) | SerumPTH(pmol/L) | Urinaryca:crratio |
|---|
| 2 | 10.6 | --- | 6.8 | 31.09 | --- | 11.50 | --- |
| 12 | 13.6 | 1.81 | 4.4 | 146.0 | 76.24 | 0.8 | --- |
| 13 | 14.0 | 1.82 | 3.5 | --- | --- | --- | 2.9 |
| 14 | 14.5 | 1.77 | 5.5 | --- | --- | --- | --- |
| 15 | 14.0 | 1.47/1.50 | 4.4 | 70.0 | --- | 1.2 | 1.4 |
| 16 | 12.2 | 1.22 | 5.0 | --- | --- | --- | --- |
| 17 | 11.9 | 1.15 | 4.2 | --- | --- | --- | 1.2 |
| 28 | 9.9 | 1.02 | 4.9 | 30.0 | 34.68 | 4.7 | 0.9 |
Normal serum levels: calcium, 8.8-10.2 mg/mL; phosphate, 3.8-6.2 mg/dL; ionised calcium, 1.0-1.25 mmol/L; 25(OH)2D3, 20-50 ng/mL; 1,25(OH)2D3, 19.6-54.3 pg/mL; PTH (parathyroid hormone), 0.95- 6.8 pmol/L; calcium creatinine ratio, <0.6.
Discussion
Hypercalcaemia may occur before or after initiation of therapy in patients with active TB and other granulomatous diseases and is due to dysregulated extrarenal production of 1,25(OH)2D3 from activated macrophages [1]. Most of the patients who develop hypercalcaemia have pulmonary TB (1, 2). Payne et al., reviewed all previously reported 12 paediatric patients and found that only 2 had extrapulmonary TB [2]. None however had tubercular meningitis (TBM) as seen in our case. The diagnosis of TBM in our patient was based on the subacute presentation, tuberculin test positivity, brain imaging findings, and high ADA levels which has a sensitivity of 94.7%, specificity 90.4%, positive predictive value 90.0 % and a negative predictive value of 95.0% for diagnosis of TBM [3].
The extra-renal overproduction of 1,25(OH)2D3 by alveolar immune cells which escapes the normal feedback control by parathyroid hormone is considered to be the underlying mechanism in TB associated hypercalcaemia [4]. The 1,25(OH)2D3 overproduction is thought to provide protection against oxidative injuries due to the nitric oxide burst from granulomatous macrophages [5]. The reason why most patients with TB do not develop hypercalcaemia is because 1,25(OH)2D3 is produced in small quantities locally and is not carried to target sites for the regulation of calcium homeostasis. This mechanism of hypercalcaemia appears to be operative in patients with pulmonary TB. However, the underlying mechanism in extrapulmonary TB, as seen in our patient, remains speculative. A recent study indicates that circulating monocytes may be an alternative source of 1-α hydroxylase that could convert 25(OH)D3 to 1,25(OH)2D3 [4]. Although 1,25(OH)2D3 produced by monocytes can act locally, these cells are carried by the blood to target tissues throughout the body. Similar to the alveolar production by macrophages, the relative contribution of monocyte source of 1,25(OH)2D3 to the total 1,25(OH)2D3 level is small in most cases of TB [4]. The elevation of circulating 1,25(OH)2D3 concentrations may lead to enhanced absorption of calcium from the gastrointestinal tract resulting in hypercalcaemia.
The risk of hypercalcaemia in TB increases when patients are provided with nutritious diets along with calcium and vitamin D supplements before the start of ATT, as happened in our case. The normal calcium levels during first week of hospitalisation in our patient indicate that hypercalcaemia was not due to the disease. But normal initial 25(OH)D3 and moderately elevated 1,25(OH)2D3 at the time of hypercalcaemia suggest that the hypercalcaemia could be secondary to disease itself. It is also possible that the 1,25(OH)2D3 concentrations were initially much higher and became somewhat suppressed after vitamin D supplementation. Nevertheless, TB associated hypercalcaemia has also been reported with normal or low levels of serum 1,25(OH)2D3 [6,7]. As hypercalcaemia developed on day 12 of vitamin D supplementation which is the usual time for 25(OH)D3 to peak after supplementation, vitamin D excess could have contributed significantly to hypercalcaemia in our patient. Another suggested mechanism is a contribution from abnormal activation of parathyroid hormone-related protein usually associated with hypercalcaemia of malignancy and sometimes found elevated in patients with sarcoidosis [7].
In patients with active TB, hypercalcaemia appears to occur more often after usual recommended supplementation of vitamin D [8]. Vitamin D deficiency (VDD) is common in patients with active TB and supplementary vitamin D in usual daily doses or high dose is often started alongwith ATT [9]. Additionally all hospitalised children are at risk of developing or exacerbating an existing VDD [10]. We practice routine supplementation of all hospitalised children with 400 IU of vitamin D which was done in the index patient also. However, in view of several reports of TB associated hypercalcaemia developing after routine supplementation or bolus doses of vitamin D, the practice of starting vitamin D and calcium supplements along with ATT is not without risk and should be reserved for those with VDD [1,2,8]. For others, close monitoring of serum calcium concentration and administration of smaller doses of vitamin D may suffice.
Conclusion
We present a rare occurrence of hypercalcaemia in an infant with extra-pulmonary tuberculosis. In the context of current recommendations on vitamin D supplementation, it is important to observe caution while initiating vitamin D and calcium supplements before anti-tubercular therapy. Close clinical and laboratory monitoring for hypercalcaemia is essential in these cases.
Normal serum levels: calcium, 8.8-10.2 mg/mL; phosphate, 3.8-6.2 mg/dL; ionised calcium, 1.0-1.25 mmol/L; 25(OH)
2D
3, 20-50 ng/mL; 1,25(OH)
2D
3, 19.6-54.3 pg/mL; PTH (parathyroid hormone), 0.95- 6.8 pmol/L; calcium creatinine ratio, <0.6.
[1]. Chan TY, Chan CH, Shek CC, The prevalence of hypercalcaemia in pulmonary and miliary tuberculosis: a longitudinal studySingapore Med J 1994 35(6):613-15. [Google Scholar]
[2]. Payne HA, Menson E, Sharland M, Bryant PA, Symptomatic hypercalcaemia in paediatric tuberculosisEur Respir Rev 2011 20(119):53-56. [Google Scholar]
[3]. Gupta BK, Bharat A, Debapriya B, Baruah H, Adenosine deaminase levels in csf of tuberculous meningitis patientsJ Clin Med Res 2010 2(5):220-24. [Google Scholar]
[4]. Tung Y-C, Ou T-T, Tsai W-C, Elevated 1-α Hydroxylase activity in monocytes from patients with active tuberculosisClin Dev Immunol 2013 2013:928138 [Google Scholar]
[5]. Chang JM, Kuo MC, Kuo HT, Hwang SJ, Tsai JC, Chen HC, 1-alpha,25-Dihydroxyvitamin D3 regulates inducible nitric oxide synthase messenger RNA expression and nitric oxide release in macrophage-like RAW 264.7 cellsJ Lab Clin Med 2004 143(1):14-22. [Google Scholar]
[6]. Ayonrinde OT, Zimmerman MJ, Ascites, hypercalcaemia, diffuse peritoneal thickening and elevated OM-MA in a fifteen-year-old girl (a case of peritoneal tuberculosis)Intern Med J 2004 34(4):216-17. [Google Scholar]
[7]. Meuthen I, Kirsch L, Saborowski F, Hypercalcaemia in florid pulmonary and cervical lymph node tuberculosisDtsch Med Wochenschr 1991 116(23):899-902. [Google Scholar]
[8]. Lavender TW, Martineau AR, Quinton R, Schwab U, Severe hypercalcaemia following vitamin D replacement for tuberculosis-associated hypovitaminosis DInt J Tuberc Lung Dis 2012 16(1):140 [Google Scholar]
[9]. Martineau AR, Old wine in new bottles: vitamin D in the treatment and prevention of tuberculosisProc Nutr Soc 2012 71(1):84-89. [Google Scholar]
[10]. Dayal D, Kumar S, Sachdeva N, Kumar R, Singh M, Singhi S, Fall in vitamin D levels during hospitalization in childrenInt J Paediatr 2014 2014:291856 [Google Scholar]