Heart rate variability (HRV) refers to the beat-to-beat fluctuations in the cardiac rhythm occurring due to modulation of the pacemaker (sinoatrial node) activity of the heart by the sympathetic and parasympathetic branches of the autonomic nervous system. HRV is considered as a standard, reliable and non-invasive means to provide a comprehensive, quantitative and qualitative evaluation of neuro-autonomic function in a continuous, beat-to-beat manner, with lower spectral value of HRV generally being considered as an indicator of poorer autonomic function [1].
Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology has provided the standards for measurement, physiological interpretation and clinical use of HRV and is followed worldwide [2]. Task force has provided normative data for time domain and frequency domain parameters of HRV for adults only. Further they have mentioned not to use the same for clinical conclusions since the data provided by them was from small number of students and not adjusted for age, sex and environment [2]. Hence, it becomes necessary to establish normative data adjusted for age, sex, and environment.
In the last decade, research on HRV has increased due to its clinical application of identifying cardiovascular autonomic imbalance during the pathogenesis of a cardiovascular disease [3]. With the documented worldwide increase in cardiovascular morbidity and mortality, detection of cardiovascular risk factors earlier in life of a person would be helpful in providing timely intervention [4]. Now-a-days, cardiovascular diseases and their risk factors are increasingly occurring at a younger age (children and adolescents) [5,6]. HRV testing can possibly play an important role in detecting the cardiovascular autonomic derangement even in children and adolescents. However, to be used clinically, normative data has to be established in this age group considering other major factors that can influence HRV such as sex [7–11], physical activity [12,13] and body mass index (BMI) [14–16]. To the best of our knowledge, there is paucity of established normative data especially for the adolescent population in India. Therefore, in the present study, we have described the normative data for HRV in the adolescent population with normal BMI, stratified based on sex and physical activity.
Materials and Methods
Study Design
The study was conducted from 28th March 2012 to 30th June 2013 as a collaborative project between the Department of Physiology, Jawaharlal Institute of Postgraduate Medical Education & Research (JIPMER), and a Central Government rural residential school (Jawahar Navodaya Vidyalaya) for the lower socio-economic students in Pondicherry, India. The study was commenced after obtaining approval from the JIPMER Scientific Advisory Committee and JIPMER Institute Ethics Committee for human studies. We present here the baseline data collected from the randomized controlled trial (Registration No. CTRI/2013/08/003897) designed to compare the effects of six months of globally recommended Structured Physical Activity with that of Unstructured Physical Activity in healthy school going adolescents.
Participants
Participants were volunteer school students (n=451; boys: 255, girls: 196) in self-reported good health in the age group of 12-17 years. Age of the participants was taken from the date of birth specified in their school records. Participants underwent a general physical examination to screen for any physical or mental ailments that can affect HRV and excluded volunteers with any co-morbidity that contraindicated exercise and/or with history of previous or current disorder that can interfere with HRV assessment and neurocognitive assessment. In girls, we excluded those with irregular cycles and HRV recording was done five days after menstruation. The recruited students (n=439; boys: 250, girls: 189) were then sub classified based on their physical activity status into non-athletes (n=360; boys: 205, girls: 155) and athletes (n=79; boys: 45, girls: 34). We have defined an athlete as a student who had represented the school at state, national or international level athletic interscholastic sport event and was undergoing supervised physical training. After explaining the study procedure, written assent from the students and written consent from their local guardian or parents were obtained.
Heart Rate Variability Recording and Analysis
The recommendations of Task Force on HRV were followed for recording short term HRV [2]. Students were asked to refrain from heavy physical activity for 24 hours and from consumption caffeinated beverages or any stimulant drinks for 12 hours prior to the recording. Students were asked to report at 8.00 am in minimal, loose clothing and after emptying their bladder. They were made to sit comfortably and the HRV recording procedure was explained to them. This enabled them to accustom to the environment with dim lighting and room temperature 24–26oC. Bioharness (Zephyr, USA), a portable, wireless data acquisition system for electrocardiogram (ECG) was applied to the student. After the student had underwent ten minutes of supine rest, lead II ECG was acquired at the rate of 200 samples/second for 10 minutes in supine rest.
After excluding the artefacts and ectopics from the RR interval series given by Bioharness, a stationary five-minute RR interval series was chosen and analysed with Kubios HRV Version 2.0 software for HRV (Bio-signal analysis Group, Finland). HRV analysis software analyses the frequency spectrum components using fast Fourier transformation and the results were given as spectral power in ms2 including very low frequency (VLF; 0.003 Hz to 0.04 Hz), low frequency (LF; 0.04 Hz to 0.15 Hz) and high frequency (HF; 0.15 Hz to0.4 Hz). Total spectral power (TP =VLF + LF + HF) and other calculated parameters like low frequency power in normalized units (LFnu = LF X 100/ (TP–VLF), high frequency power in normalized units (HFnu = HF X 100/ (TP–VLF) and LF/HF ratio (ratio of LF power to HF power) were obtained. HRV analysis software analyses the RR interval time domain components and the results were given as standard deviation of RR intervals (SDNN), square root of the mean of the sum of the squares of differences between adjacent RR intervals (RMSSD), adjacent RR interval differing more than 50 ms (NN50) .
Physiological Correlates
Both parasympathetic and sympathetic activity modulates heart rate by influencing the pulse interval (RR interval) [17]. Hence, to quantify autonomic activity, variability in the pulse interval has to be considered rather than heart rate [18]. Effect of change in parasympathetic outflow to the heart is seen very rapidly as a change in heart rate, usually within 400 ms, and hence it can control heart rate on a beat-to-beat basis [19]. Hence, the beat-to-beat changes determined by time domain parameters of HRV analysis signify resting parasympathetic activity. The effect of change in sympathetic outflow to the heart usually takes more than 5s to change the heart rate [18]. Hence, high frequency component in the RR tachogram is considered to be due to resting parasympathetic activity and low frequency component is considered mainly to be due to resting sympathetic activity.
The total power provides the overall heart rate variability observed in the recording. Resting parasympathetic activity is the major contributor for HF power, HFnu, SDNN, RMSSD, and NN50. While LFnu reflects sympathetic activity, LF component is predominantly due to resting sympathetic activity with some influence from resting parasympathetic activity. Physiological explanation for VLF component is ill defined and it cannot be interpreted based on short-term HRV recordings and so is not discussed in this manuscript. LFnu and HFnu represent controlled and balanced resting activity of sympathetic and parasympathetic nervous system and LF/HF ratio indicates sympatho-vagal autonomic balance [2].
Statistical Analysis
Data were checked for normal distribution and none of the parameters passed the Kolmogorov-Smirnov test for normality. Hence data were represented in median with 25th and the 75th percentiles along with minimum and maximum values. Comparison between the boys and girls and between athletes and non-athletes were done using Mann Whitney U test.
Results and Discussion
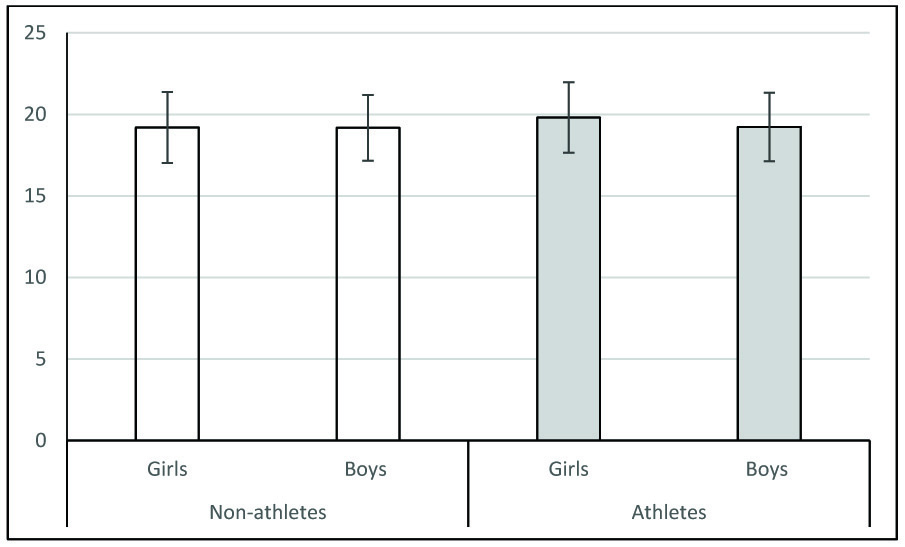
The objective of the present study is to establish sex and physical activity stratified normative data of HRV in apparently healthy adolescent population with normal BMI. Sex stratified normative data for non-athletes and athletes are provided in [Table/Fig-1,2] respectively. As mentioned earlier age, sex, BMI and physical activity are the main factors that can influence HRV. It has been shown that as age increases, HRV reduces [9]. In our study we have considered only a narrow age group i.e. students with age between 12 and 17 years. Hence, we consider that the normative data provided here to be relatively free from the variability due to age. BMI is another factor known to negatively influence HRV. Obesity increases sympathetic activity and decreases parasympathetic activity [20]. In the present study, BMI of the students were within normal range for given age and sex according to Indian standards [21]. Hence, the given normative data is for normal BMI adolescent population. Further, even in non-obese healthy adults BMI is negatively correlated with parasympathetic activity [15]. In our study, BMI was comparable between the groups [Table/Fig-3]. Hence, we consider that the differences observed between girls and boys, and between non-athletes and athletes as discussed below to be relatively free from the influence of BMI.
Sex stratified normative data of heart rate variability in non–athletes.
| Parameters | Girls | Boys | P value |
|---|
| Median | Percentiles | Minimum | Maximum | Median | Percentiles | Minimum | Maximum |
|---|
| 25 | 75 | | | | 25 | 75 | | | |
|---|
| Mean heart rate | 89.29 | 82.86 | 98.81 | 64.44 | 120.90 | 82.51 | 74.53 | 92.36 | 51.59 | 119.26 | <.001 |
| SDNN (ms) | 66.35 | 46.00 | 86.78 | 9.40 | 193.80 | 63.20 | 48.40 | 84.60 | 12.80 | 374.50 | .552 |
| RMSSD (ms) | 69.00 | 49.05 | 99.60 | 12.80 | 256.30 | 58.70 | 44.80 | 88.20 | 9.10 | 350.40 | .024 |
| NN50 count | 137.50 | 79.75 | 180.00 | 8.00 | 268.00 | 116.00 | 68.00 | 158.50 | 1.00 | 272.00 | .020 |
| LF (ms2) | 1015.00 | 683.25 | 1782.00 | 208.00 | 6374.00 | 945.00 | 571.00 | 1402.00 | 169.00 | 7236.00 | .038 |
| HF (ms2) | 1324.00 | 758.25 | 2465.25 | 240.00 | 7896.00 | 988.00 | 513.50 | 1940.00 | 27.00 | 8514.00 | .001 |
| Total Power (ms2) | 3374.50 | 2324.75 | 5419.00 | 894.00 | 21250.00 | 2757.00 | 1953.50 | 4594.50 | 544.00 | 20350.00 | .005 |
| LF/HF ratio | 0.83 | 0.60 | 1.17 | 0.08 | 3.79 | 0.91 | 0.55 | 1.90 | 0.10 | 19.87 | .088 |
| LF norm (n.u.) | 45.44 | 37.33 | 53.94 | 7.08 | 79.11 | 47.63 | 35.49 | 65.47 | 8.95 | 95.21 | .088 |
| HF norm (n.u.) | 54.56 | 46.06 | 62.67 | 20.89 | 92.92 | 52.37 | 34.53 | 64.51 | 4.79 | 91.05 | .088 |
SDNN: Standard deviation of all NN intervals; RMSSD: Square root of mean of the sum of the squares of differences between adjacent NN intervals; NN50 count: Number of pairs of adjacent NN intervals differing by more than 50 ms in entire recording; Total power: The variance of NN intervals over the temporal segment; LF: Power in low frequency range (0.04-0.15 Hz); HF: Power in high frequency range (0.15-0.4 Hz); LF norm: LF power in normalized units (LF/(TP-VLF)*100); HF norm: HF power in normalized units (HF/(TP-VLF)*100); LF/HF ratio: Ratio LF (ms2)/ HF (ms2). Comparison between girls and boys is done using Mann-Whitney U-test
Sex stratified normative data of heart rate variability in athletes.
| Parameters | Girls | Boys | P value |
|---|
| Median | Percentiles | Minimum | Maximum | Median | Percentiles | Minimum | Maximum |
|---|
| 25 | 75 | | | | 25 | 75 | | |
|---|
| Mean heart rate | 80.67 | 72.48 | 89.25 | 60.83 | 109.60 | 72.27 | 66.37 | 80.41 | 53.72 | 106.85 | .003 |
| SDNN (ms) | 113.00 | 100.60 | 132.00 | 66.40 | 225.90 | 94.20 | 82.20 | 117.75 | 22.80 | 205.50 | .006 |
| RMSSD (ms) | 94.90 | 77.00 | 119.10 | 45.30 | 211.00 | 100.30 | 77.55 | 125.05 | 18.30 | 265.90 | .877 |
| NN50 count | 137.00 | 93.00 | 174.00 | 42.00 | 260.00 | 156.00 | 109.50 | 191.00 | 2.00 | 268.00 | .186 |
| LF (ms2) | 1465.00 | 1090.00 | 1732.25 | 782.00 | 6263.00 | 1211.00 | 988.88 | 1800.25 | 501.00 | 4906.00 | .128 |
| HF (ms2) | 2409.00 | 1874.00 | 3261.50 | 1256.00 | 8250.00 | 2219.00 | 1581.50 | 3333.50 | 892.00 | 7364.00 | .635 |
| Total Power (ms2) | 5202.00 | 3889.00 | 6390.50 | 2869.00 | 16428.00 | 5273.00 | 3757.00 | 7264.50 | 1732.00 | 15385.00 | .712 |
| LF/HF ratio | 0.63 | 0.45 | 0.75 | 0.29 | 1.81 | 0.59 | 0.44 | 0.71 | 0.15 | 1.11 | .248 |
| LF norm (n.u.) | 38.59 | 31.17 | 42.98 | 22.20 | 64.39 | 37.10 | 30.45 | 41.66 | 13.27 | 52.61 | .248 |
| HF norm (n.u.) | 61.41 | 57.02 | 68.83 | 35.61 | 77.80 | 62.90 | 58.34 | 69.55 | 47.39 | 86.73 | .248 |
SDNN: Standard deviation of all NN intervals; RMSSD: Square root of mean of the sum of the squares of differences between adjacent NN intervals; NN50 count: Number of pairs of adjacent NN intervals differing by more than 50 ms in entire recording; Total power: The variance of NN intervals over the temporal segment; LF: Power in low frequency range (0.04-0.15 Hz); HF: Power in high frequency range (0.15-0.4 Hz); LF norm: LF power in normalized units (LF/(TP-VLF)*100); HF norm: HF power in normalized units (HF/(TP-VLF)*100); LF/HF ratio: Ratio LF (ms2)/ HF (ms2). Comparison between girls and boys is done using Mann-Whitney U test.
Comparison body mass index between groups.
Comparison between groups were done using student t-test. Data are expressed Mean ± SD. BMI was comparable between the groups. * p< .05,** p<0.01, ***p< .001
According to the Task Force, spectral analysis of HRV is more reliable than the time domain analysis in a 5 min recording of ECG [2]. The time domain analysis data provided by them is based on 24 hour ECG recording. Hence for data comparison, we considered only the HRV spectral analysis data provided by the Task Force as it is based on 5-min recording. Total power seems to be similar between the Task Force data and the data for non-athletes of both sexes in our study. However, athletes in our study seem to have higher total boring compared to the Task Force data. The major difference between Task Force data and our data is that, irrespective of sex and physical activity (athlete/non-athlete), HF power was more and LF power was less in our data. This trend is further reflected in the values of LFnu, HFnu and LF/HF ratio. Then, we compared our data with the data on healthy Adults (both sex) previously published from our lab [22]. We observed that indicators of parasympathetic activity such as SDNN, RMSSD and NN50 count seems to be more in our data in non-athletes of both sex. The increase was even more when we considered the athletes of both sex. Even though the LFnu and HFnu values of non-athletes were same as that given by Thiyagarajan et al., [22]. LF/HF ratio seems to be lower in both non-athletes and athletes of both sexes in our study. Based on the above observations we conclude that resting parasympathetic activity is more in adolescents as compared to adults.
The other major factor which can influence HRV is sex. In adults, females were considered to have better HRV than males in terms of increased parasympathetic activity and decreased sympathetic activity [23]. In the present study, we have observed that indicators of parasympathetic activity like total power, HF, RMSSD and NN50 were significantly more in girls than boys. We observed that LF, an indicator of sympathetic activity, was more in girls than boys [Table/Fig-1]. Since LF power is not only due to sympathetic activity and has some parasympathetic component too, LFnu is considered to be a better indicator of resting sympathetic activity than LF power. When we consider LFnu and HFnu, girls show higher relative parasympathetic activity and lesser relative sympathetic activity when compared to boys. However, the results were not statistically significant. LF/HF ratio was less in girls than boys but not statistically significant [Table/Fig-1]. The better HRV in females as compared to males is considered to be because of difference in their hormonal profile; testosterone increases sympathetic activity and estrogen decreases sympathetic activity [24–26]. We hypothesize that the lack of significant difference between girls and boys in some of the parameters in our study may be because of less optimal sex hormones levels in adolescent age group. Based on the above findings we conclude that in non-athletes, girls have increased parasympathetic activity than boys. Different values between girls and boys justify sex stratification of normative data in our study. In contrast to the above finding, we observed that except for mean HR and SDNN, all the other time domain and frequency domain parameters were comparable between girls and boys in athletes group [Table/Fig-2]. Significant differences between girls and boys in non-athletes but not in athletes lead us to hypothesize that athletic level physical training in boys could have improved their HRV similar to that of girls. However, the major limitation of this argument is that athlete girls also had physical training and we except it to improve their HRV. Possible arguments may be that the level of training is more in boys (which we have not measured) or the physical activity may have different influence on girls and boys (which requires further research).
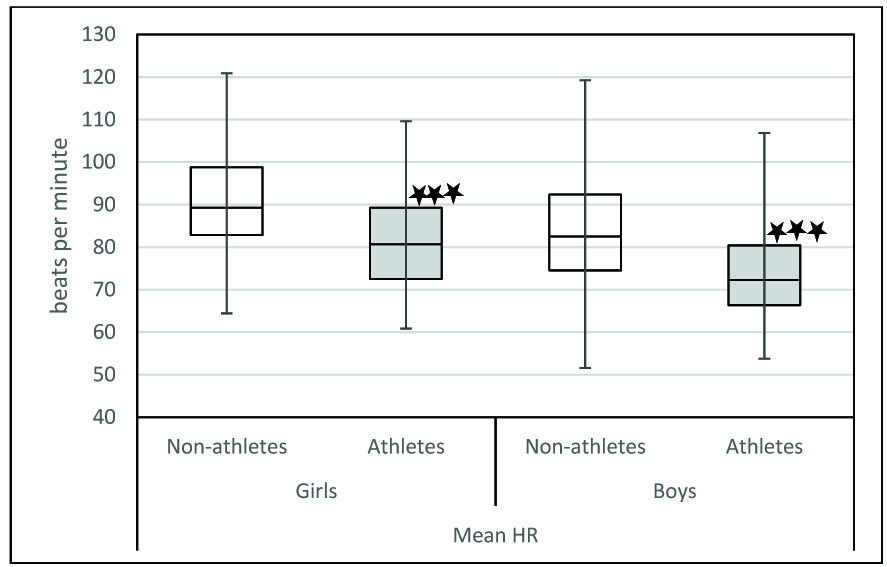
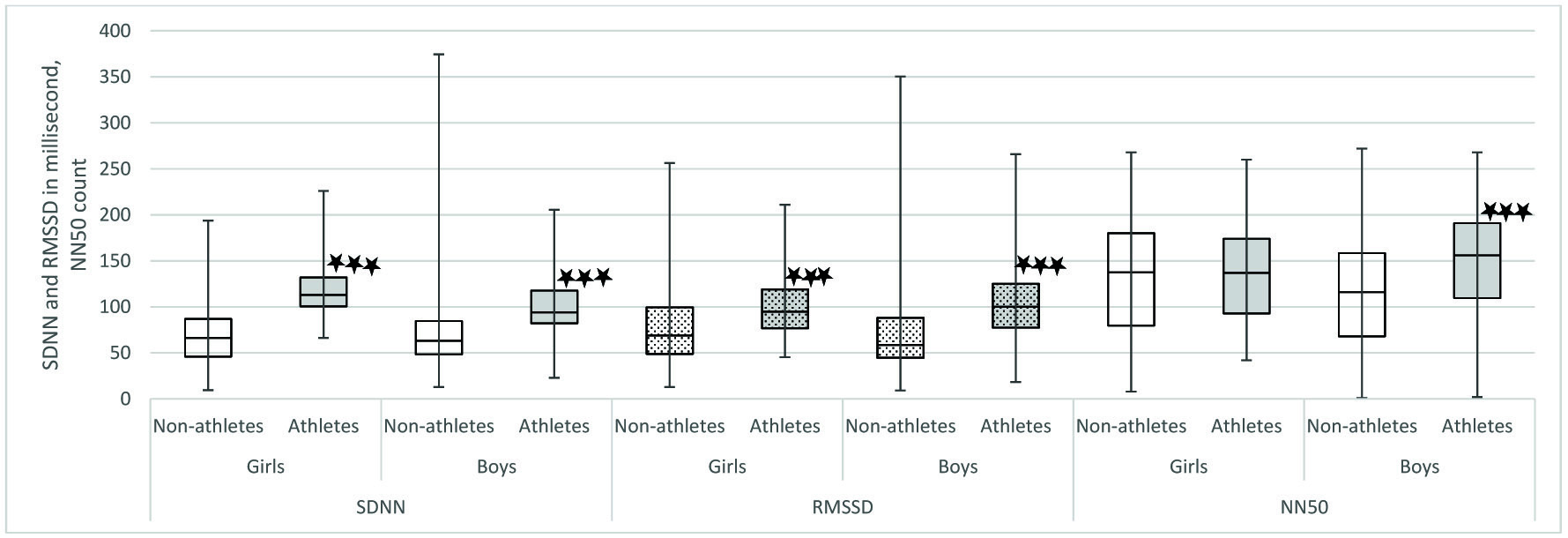
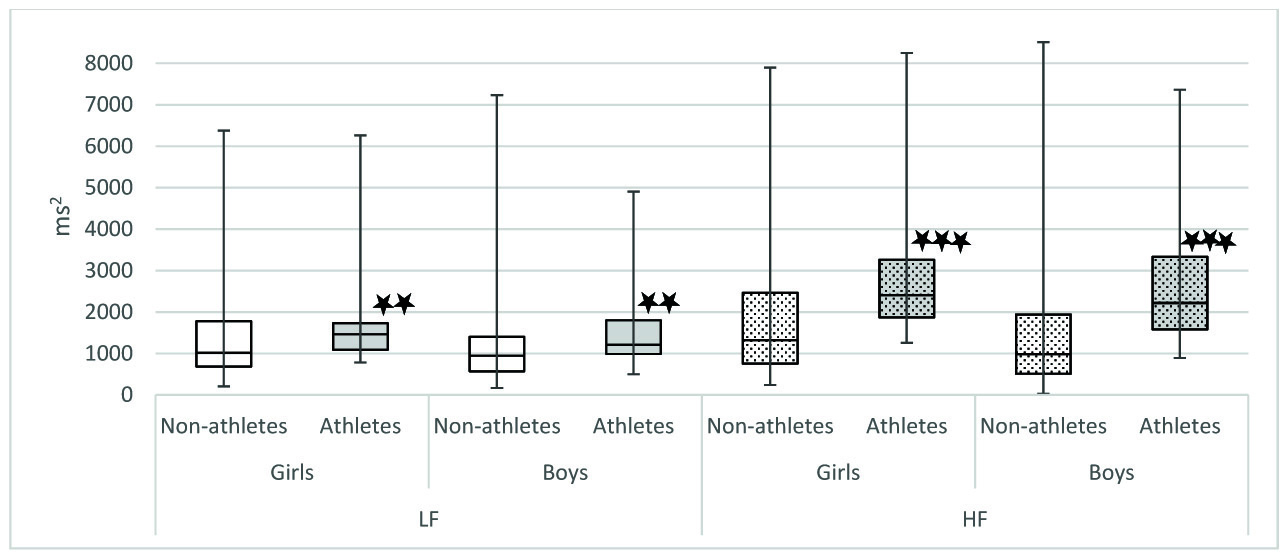
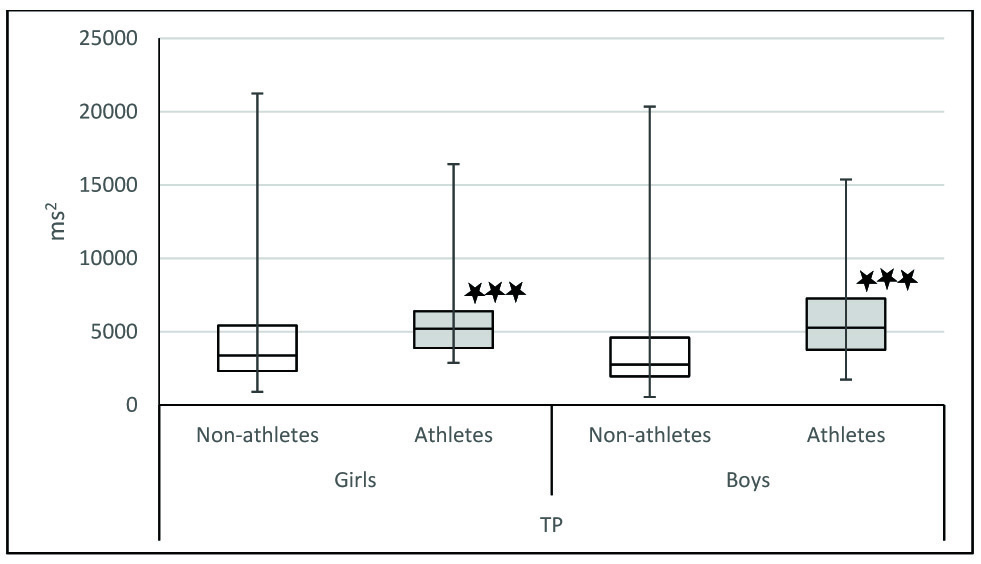
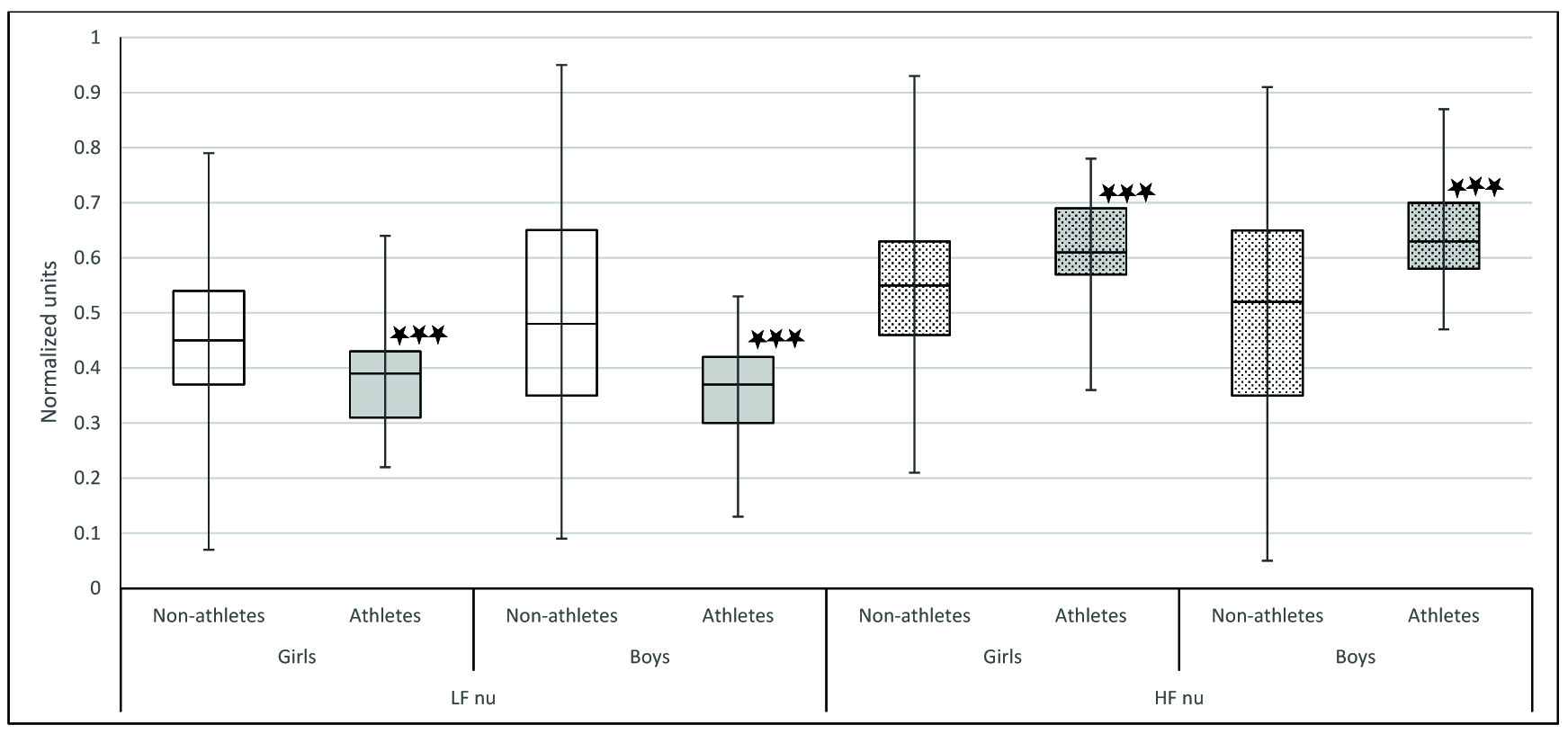
In our study, we observed that when we compared athletes and non-athletes, the physiological parameters were significantly different in both sexes. Mean HR was significantly lower in athletes of both sex [Table/Fig-4]. It has been proven that athletes have decreased heart rate because of increased stroke volume as a result of their cardiovascular training. The decrease in heart rate may also be due to increased resting parasympathetic activity. Indicators of parasympathetic activity such as SDNN, RMSSD, NN50, HF power, HFnu were significantly more in athletes of both sex [Table/Fig-5,6 and 7]. LFnu was significantly lower in athletes of both genders indicating relatively decreased sympathetic activity [Table/Fig-7]. Further, overall HRV was more in athletes of both sexes based on significantly higher total power [Table/Fig-8]. Based on the above findings, we conclude that irrespective of sex, athletic level training had positive influence on heart rate variability in terms of increased resting parasympathetic activity and decreased sympathetic activity. Our results are in accordance with the previous studies on the effect of exercise on heart rate variability [27,28]. Athletic level training can bring about change in their BMI which may be the reason for the significant change in their HRV. In our study the BMI was comparable. We have discussed the body composition difference between athletes and non-athletes in our previous publications [29,30]. We also observed that in our study influence of physical activity on HRV more than the influence of sex on HRV.
Comparison of Mean HR between Athletes and non-athletes.
Comparison between athletes and non-athletes was done using Mann-Whitney U test. * p< .05,** p<0.01, ***p< .001
Comparison of time domain parameters between Athletes and non-athletes. SDNN: Standard deviation of all NN intervals; RMSSD: Square root of mean of the sum of the squares of differences between adjacent NN intervals; NN50 count: Number of pairs of adjacent NN intervals differing by more than 50 ms in entire recording. Comparison of time domain parameters was done between athletes and non-athletes using Mann-Whitney U test. * p< .05,** p<0.01, ***p< .001
Comparison of frequency domain parameters between Athletes and non-athletes. LF: Power in low frequency range (0.04-0.15 Hz); HF: Power in high frequency range (0.15-0.4 Hz); Comparison of frequency domain parameters between athletes and non-athletes was done using Mann-Whitney U-test; * p< .05,** p<0.01, ***p< .001
Comparison of total power between athletes and non-athletes.
TP: Total power; Comparison of frequency domain parameters between athletes and non-athletes was done using Mann-Whitney U test; * p< .05,** p<0.01, ***p< .001
Comparison of LF nu and HF nu between Athletes and non-athletes. LF norm: LF power in normalized units (LF/(TP-VLF)*100); HF norm: HF power in normalized units (HF/(TP-VLF)*100). Comparison of LF nu and HF nu between athletes and non-athletes was done using Mann-Whitney U test; * p< .05,** p<0.01, ***p< .001
Conclusion
We have given sex and physical activity stratified HRV normative data for normal BMI adolescent in the age between 12-17 years. Data given below are expressed as median with interquartile range (Median(IQR)) in the following order: non-athlete girls, non-athlete boys, athlete girls and athlete boys.
Time domain indices: SDNN- 66.35 (40.78), 63.20 (36.20), 113.00 (31.40) and 94.20 (35.55); RMSSD – 69.00 (50.55), 58.70 (43.40), 94.90 (42.10) and 100.30 (47.50); NN50- 137.50 (100.25), 116.00 (90.50), 137.00 (81.00) and 156.00 (81.50). The frequency domain indices – LF power 1015.00 (1098.75), 945.00 (831.00), 1465 (642.25), and 1211.00 (811.37); HF power – 1324.00 (1707.00), 988.00 (1426.50), 2409.00 (1387.50), and 2219.00 (1752.00); Total power – 3374.50 (3094.25), 2757.00 (2641.00), 5202.00 (2501.50) and 5273.00 (3507.50); LFnu – 45.44 (16.61), 47.63 (29.98), 38.59 (11.81) and 37.10 (11.21); HFnu – 54.56 (16.61), 52.37 (29.98), 61.41 (11.81) and 62.90 (11.21).
Strengths and Limitations
To the best of our knowledge, no study has been done in India to establish sex and physical activity stratified normative data of heart rate variability in adolescents. Students had routine physical activity is school for at least one hour that may have influenced the autonomic status.
Generalizability
Students were from a controlled environment (residential, food and physical activity) and more or less the same socio-economic status. So, generalizability of this data may be minimal but can be added to the general pool.
SDNN: Standard deviation of all NN intervals; RMSSD: Square root of mean of the sum of the squares of differences between adjacent NN intervals; NN50 count: Number of pairs of adjacent NN intervals differing by more than 50 ms in entire recording; Total power: The variance of NN intervals over the temporal segment; LF: Power in low frequency range (0.04-0.15 Hz); HF: Power in high frequency range (0.15-0.4 Hz); LF norm: LF power in normalized units (LF/(TP-VLF)*100); HF norm: HF power in normalized units (HF/(TP-VLF)*100); LF/HF ratio: Ratio LF (ms2)/ HF (ms2). Comparison between girls and boys is done using Mann-Whitney U-test
SDNN: Standard deviation of all NN intervals; RMSSD: Square root of mean of the sum of the squares of differences between adjacent NN intervals; NN50 count: Number of pairs of adjacent NN intervals differing by more than 50 ms in entire recording; Total power: The variance of NN intervals over the temporal segment; LF: Power in low frequency range (0.04-0.15 Hz); HF: Power in high frequency range (0.15-0.4 Hz); LF norm: LF power in normalized units (LF/(TP-VLF)*100); HF norm: HF power in normalized units (HF/(TP-VLF)*100); LF/HF ratio: Ratio LF (ms2)/ HF (ms2). Comparison between girls and boys is done using Mann-Whitney U test.