Approximately 15% of married couples have infertility problems. Twenty percent of them originate from men, 30%-40% originate from both men and women. So, one half of the infertility couples have male-factor associated infertility [1].
Assessment of sperm morphology is the most important criterion for determining the quality of a semen sample. The goal of identifying an ideal technique for sperm staining is to ease the visualization of cells and provide a better identification of the abnormalities. This is an important factor for successful fertilization and early embryonic development in assisted reproductive techniques [2].
The accuracy of sperm morphology assessment depends on careful smear preparation, fixation and staining since these procedures can affect the sperm dimensions significantly.
Many staining methods such as Haematoxylin & Eosin, Giemsa, Papanicolau, Eosin- Nigrosin and Leishmann have been used for sperm staining. Some of the commercially available stains such as Shorr, Janus Green and Sperm Blue are too expensive to be routinely used in developing countries like India [3].
A few stains may cause a slight change in the measurement values of spermatozoa because fixatives may shrink the cells a little [4]. This creates a need for an ideal, simple, and cost effective staining method which also does not alter the sperm morphology for an accurate evaluation of the same. Sensing this need, we employed four different simple and affordable staining methods in this study and compared the results to determine the ideal staining method to accurately evaluate the sperm morphology which satisfies all these criteria.
Material and Methods
In this prospective study between October 2014 to December 2014, semen samples were collected from 62 healthy men of reproductive age group at the central laboratory attached to the Department of Pathology, Sri Adichuchanagiri Institute of Medical sciences, B. G. Nagar, India.
Initially, wet mount preparations of the semen samples were studied, followed by assessment of sperm count on improved Neubauer’s chamber.
Four smears were prepared from the collected samples, three of which were dried at room temperature for staining by H&E, Giemsa and Eosin-Nigrosin stains. The fourth smear was fixed in ethyl alcohol for staining by rapid Papanicolau stain. All the smears were analysed by four independent observers.
Inclusion Criteria
Healthy men in the reproductive age group.
Properly prepared, fixed and evenly stained smears are included.
Exclusion Criteria
Samples from subjects who have not adhered to the required period of sexual abstinence (2-7 days).
Subjects with significant co-morbidities like diabetes mellitus and hypertension.
Results
Semen samples were collected from 62 healthy men in the reproductive age group at our tertiary care centre. General physical examination of all the subjects was carried out and no co-morbidities were found. The samples collected were allowed a liquefaction time of minimum 30 minutes and maximum of 1 hour at a temperature of 37°C. Wet mount preparations were studied and the sperm count was assessed by the improved Neubauer’s chamber and the following observations were made [Table/Fig-1].
Sperm counts of the 62 study subjects
| SPERM MORPHOLOGY | NUMBER OF PATIENTS (n=62) | PERCENTAGE |
|---|
| Normozoospeermia | 28 | 45.16% |
| Oligozoospermia | 17 | 27.42% |
| Necrozoospermia | 05 | 8.06% |
| Teratozoospermia | 12 | 19.35% |
Four smears were prepared from each of the samples, three of which were air-dried to be stained with H&E, Giemsa and Eosin-Nigrosin and one was fixed in ethyl alcohol for staining with rapid Papanicolau stain.
Assessment of morphology was conducted by four different observers including, a Professor and Head of Department of Pathology with 29 years of experience, an Associate Professor of Pathology with 7 years of experience, an Assistant Professor of Pathology with 3 years of experience and a Junior Resident with 2 years of experience in semen analysis. Every observer was required to independently assess the four morphological components of the sperm namely acrosome, head, middle piece and tail of the 62 subjects which were separately stained by Haematoxylin and Eosin, Giemsa, Eosin-Nigrosin and rapid Papanicolau and grade the clarity of sperm morphology under four categories namely-Very clear, Clear, Not clear and Pale or Poorly stained by adopting a scoring system formulated by our research team [Table/Fig-2] and the results were tabulated [Table/Fig-3]. The stained smears were examined under the light microscope and the following observations were made keeping the Kruger’s Strict morphology method as the standard criterion.
Scoring system formulated and utilized by the research team
| CLARITY | SCORE |
|---|
| Very clear | 3 |
| Clear | 2 |
| Not clear | 1 |
| Pale/ poorly stained | 0 |
Tabulated results of the 62 subjects by four independent observers using the scoring system formulated by our research team
| Stain used | Head | Acrosome | Middle Piece | Tail |
|---|
| A | B | C | D | A | B | C | D | A | B | C | D | A | B | C | D |
|---|
| Hematoxylin & Eosin | 186 | 184 | 186 | 185 | 186 | 186 | 185 | 185 | 61 | 62 | 62 | 61 | 124 | 123 | 123 | 123 |
| Giemsa | 186 | 186 | 185 | 185 | 124 | 123 | 124 | 123 | 62 | 61 | 61 | 62 | 62 | 62 | 61 | 61 |
| Eosin-Nigrosin | 123 | 124 | 124 | 123 | 124 | 123 | 124 | 123 | 15 | 16 | 15 | 14 | 14 | 16 | 15 | 16 |
| Rapid Papanicolau | 186 | 185 | 186 | 185 | 185 | 186 | 186 | 185 | 124 | 123 | 123 | 122 | 124 | 123 | 123 | 123 |
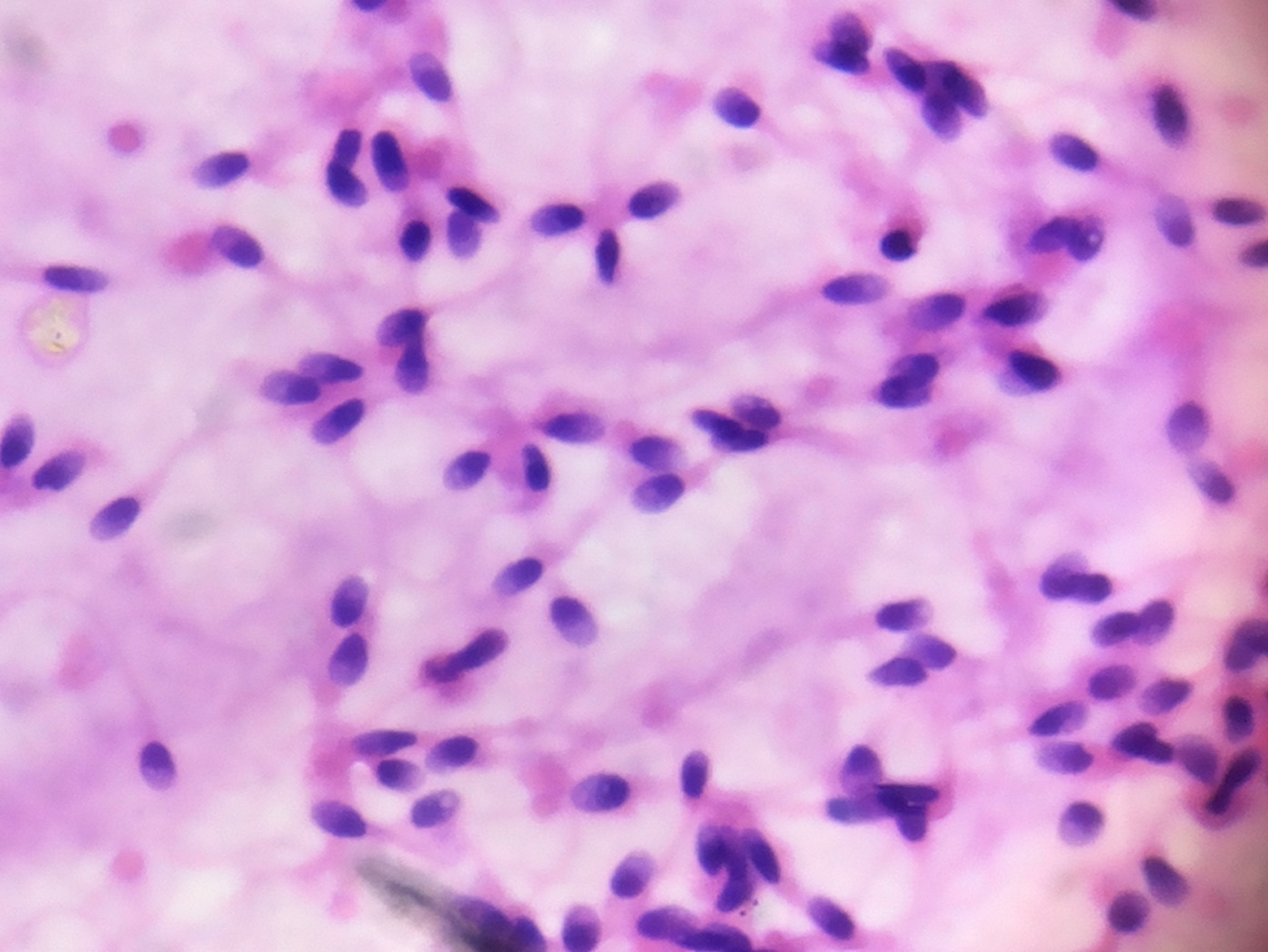
The acrosomal morphology was extremely clear on smears studied using Harris’ Haematoxylin and Eosin Y stain. The stain was uniformly distributed on the head with dark purple condensation. The middle piece and tail morphology were clearly identified as well which enabled easy evaluation of the morphological detail.
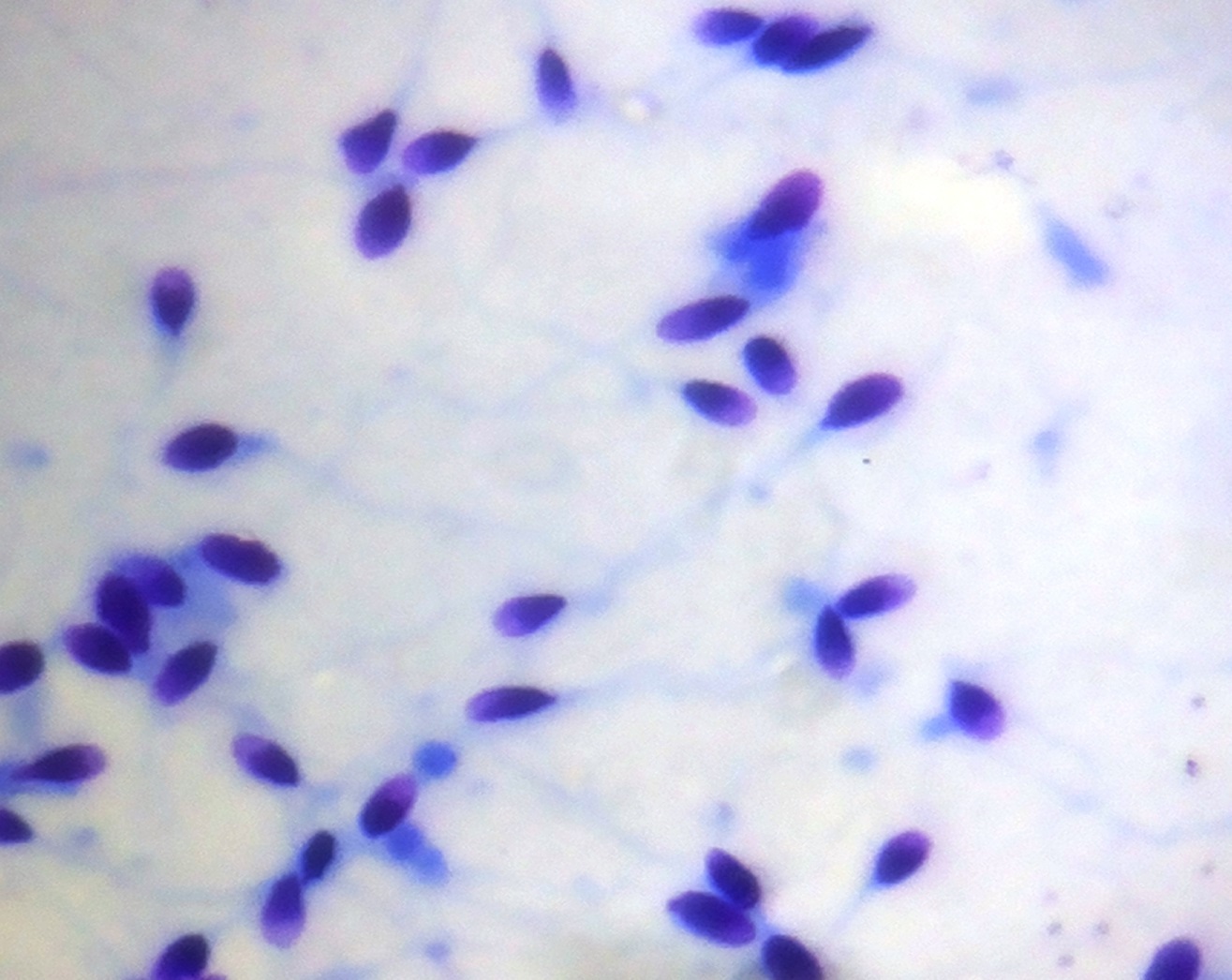
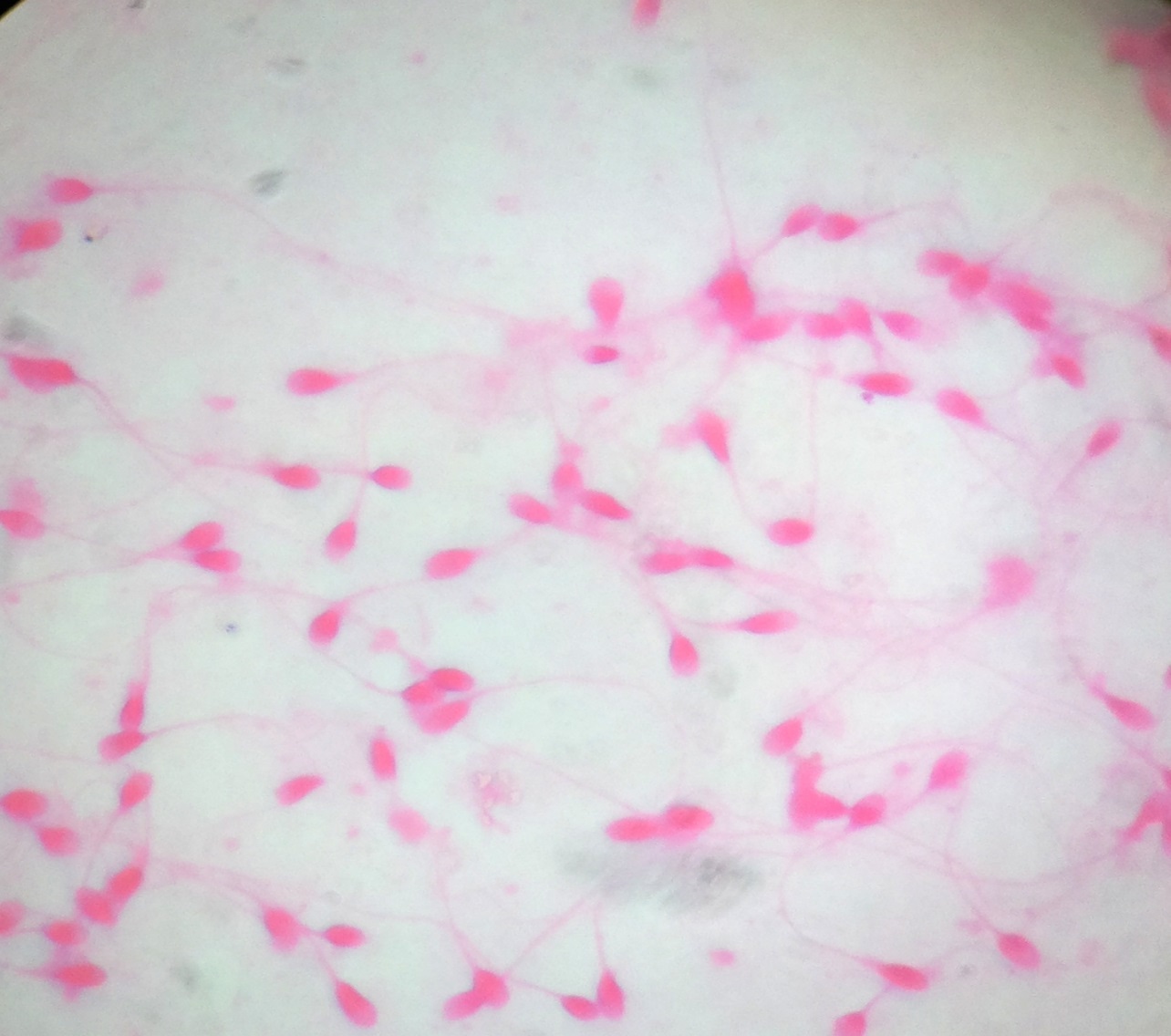
The spermatozoa stained using Giemsa took up a dark blue purple hue. The acrosomal condensation and the head morphology were seen very clearly but the middle piece and the tail were not. In Giemsa stain the spermatozoa which stained pink in the head was an additional finding. Eosin-Nigrosin stain was used for distinction between live and dead sperms. The stain is taken up by dead sperms since viable cells with intact cell membrane do not take up the stain and appear white. The head of non-viable sperms with damaged cell membrane take up the dye and were stained pink. Our results showed similar findings [Table/Fig-4].
Results of consensus of sperm morphology assessment using various staining techniques
| Staining Technique | Acrosome | Head | Middle Piece | Tail |
|---|
| Hematoxylin & Eosin | Very clear | Very clear | Not clear | Clear |
| Giemsa | Very clear | Clear | Not clear | Not clear |
| Eosin-Nigrosin | Clear | Clear | Pale | Pale |
| Rapid Papanicolau | Very clear | Very clear | Clear | Clear |
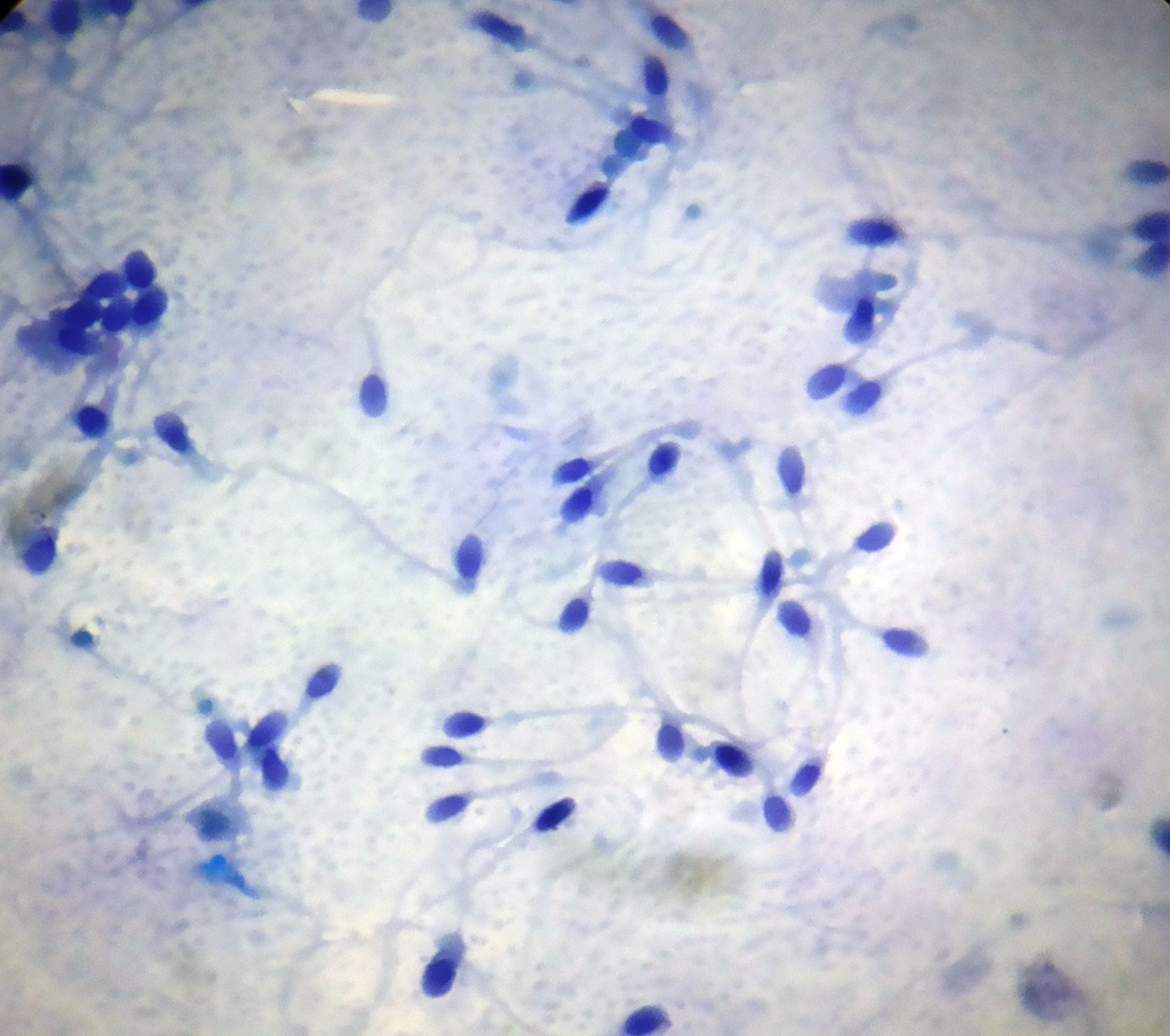
The acrosomal condensation and the head morphology were very clearly seen on rapid Papanicolau stained smears. The middle piece and tail morphology were clearly appreciable. Additionally, this stain helped in the separation of immature germ cells.
Discussion
Assessment of sperm morphology as a component of semen analysis is one of the most important steps in the evaluation of male infertility. Morphology of spermatozoa is a vital criterion for successful fertilization and early embryonic development in assisted reproductive techniques such as Invitro Fertilization (IVF), Gamete Intra Fallopian Tube Transfer (GIFT) and Intra Uterine Insemination (IUI) [5].
The most important morphological classifications for spermatozoa are Final Tygerberg Classification Criteria described by Kruger in 1986 and the new WHO semen analysis parameters of 2010 [Table/Fig-5]. As described by Kruger, the head, neck, middle piece and tail sections of the sperm should have no abnormalities, the head should have smooth and oval boundaries with an acrosomal coating of 70%. The sperm head should be 4-5 μm long and 2.5-3.5μm wide. The average length of the tail should be 45 μm. The middle part must be thin and long (1.5 times the length of the head) with a width less than 1 mm. The tail should be thinner than the middle piece, flat, not wrinkled and should not contain broken parts or cytoplasmic debris [6–8].
Normal values for semen parameters according to new WHO semen analysis parameters [7]
| PARAMETERS | REFERENCE VALUEVALUES |
|---|
| Volume | 1.5 mL |
| pH | ± 7.2 |
| Sperm concentration | ≥15 X 106 spermatozoa/mL |
| Total sperm number | ≥40 X 106 spermatozoa per ejaculate or more |
| Motility | ≥50% total motility or .32% progressive motility |
| Morphology | WHO: >4% normal |
| Vitality | ≥50% or more live, i.e. excluding dye |
One of the criteria for a good staining method is that, processes involved such as fixation should cause as little change to sperm morphology as possible [9].
The study by Emine Aksoy et al., indicates that Papanicolaou stain is the standard stain for human sperm morphology assessment by both the WHO and the Tygerberg Strict Criteria. Despite the extensive application of PAP for staining human sperms and its suitability for automated sperm morphology analysis, the procedure involves processing through more than 20 steps and more than 12 different chemical solutions. It is not only time consuming, but alcohol fixation and alcohol or xylene dehydration may cause cell shrinkage [4].
The Diff- Quick stain and Giemsa staining procedures are rapid methods for evaluating sperm morphology and sperm chromatin status. They involve fixation followed by staining with two staining solutions. Despite the findings that Diff- Quick causes sperm swelling and background staining, it is recommended by WHO for human sperm morphology assessment [10].
Eosin-Nigrosin staining developed for “Live-dead” staining of sperm also has been used to assess sperm morphology for many animal species. However, this stain does not clearly differentiate the various components of the sperm [4]. The present study has been undertaken for comparative assessment of morphology of sperms by using Haematoxylin and Eosin, Giemsa, Rapid Papanicolaou stain and Eosin-Nigrosin stains [Table/Fig-6,7,8,9 and 10].
Spermatozoa stained by Haematoxylin & Eosin, showing very clear morphology of head of sperms (1000X)
Spermatozoa stained by Giemsa showing very clear morphology of head but middle piece & tail are not visible (1000X)
Smear from a subject with necrozoospermia showing dead sperms which appear pink on eosin stain, having taken up the dye due to damaged cell membrane (1000X)
Rapid Papanicolau stain showing overall clear morphology of sperms (1000X)
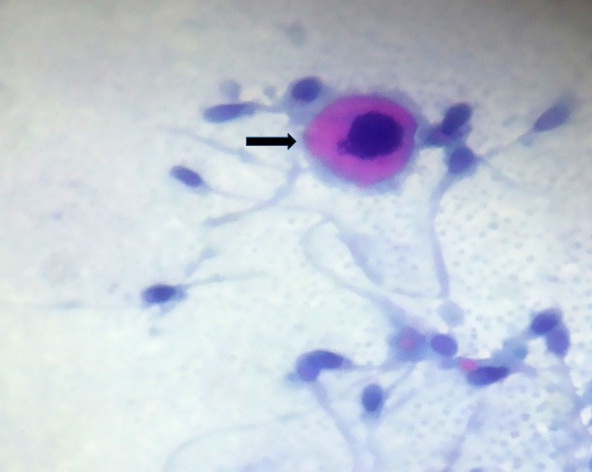
An additional finding in Rapid PAP stain was identification of immature germ cells (Black arrow), (1000X)
Of all the seminal parameters such as concentration, motility and morphology, one of the most powerful indicators of both invitro and invivo fertility remains the morphology. Although the importance of this parameter has been recognised, limitations such as lack of standardization in preparation, evaluation and staining techniques, the true potential of this parameter has not been tapped. The advent of Computer Aided Semen Analysis (CASA) has eased the process of evaluation of sperm morphology but rural tertiary care centres like ours still rely on manual microscopic examination for determination of sperm morphology. Hence, assessment of sperm morphology with an ideal, simple and cost-effective stain becomes vital and pertinent. With the increase in number of Artificial Reproduction Techniques which have come into vogue such as Invitro Fertilization (IVF), Gamete Intra-Fallopian Tube Transfer (GIFT), Intra Cytoplasmic Sperm Injection (ICSI), Intra Uterine Insemination (IUI) and Intracytoplamic Morphologically selected Sperm Injection (IMSI), accurate assessment of sperm morphology is quintessential even in the centres with modern and advanced technologies [4,5].
Morphologically, the sperm is divided in to three main parts-head, middle piece and tail. The head is capped by an acrosome which should ideally occupy 40-70% of the total head area. The acrosome contains enzymes required for penetration of the oocyte and thus plays a vital role during fertilization. The middle piece is packed with mitochondria which provide the energy required for movement of the sperm [6].
Many staining techniques have been employed for staining spermatozoa. Garcia-Herreros et al., concluded that Haematoxylin is the best staining method for the evaluation of sperm heads. Our observations were concurrent with this study regarding to staining of sperm heads [11].
Tartaglione and Ritta observed that Giemsa stain was ideal for sperm morphology evaluation since it contributed to assessment of good quality semen and favourable result in IVF. We observed that even though the morphology of the sperm head was very clear by Giemsa, the middle piece and tail morphology were not clear and thus not compatible with the other studies [12].
Eosin-nigrosin stain is used to identify dead sperms and calculate the percentage of dead and live sperms in cases of oligozoospermia and necrozoospermia. The dead sperms have a damaged cell membrane and thus take up the pink stain whereas, live sperms which have an intact cell membrane appear white. This is especially useful in cases where the subjects and the treating physicians are in a dilemma whether to adopt Artificial Reproductive Techniques or not [8].
Papanicolau stain was regarded as the best stain for assessment of sperm morphology in the study by Emine Aksoy et al., However, the staining process was considered to be time consuming [4]. In our study, we adopted the use of rapid Papanicolau stain which includes a nuclear stain and a cytoplasmic stain. The smears have to be placed in the aforementioned stains for a duration of 90 seconds each, which would therefore mean a turnaround time of less than 5 minutes per smear which took care of the limitation of excessive time factor which was the major drawback highlighted by the study by Emine Aksoy et al., The morphology of the head and acrosome were very clear and the middle piece and tail were clearly seen on rapid Papanicolau stained smears.
Limitation
The only limitation that we observed in our study was that, these observations were subjective and discrepancies and differences of opinions are possible owing to inter- observer variations.
Conclusion
Rapid Papanicolau stain was found to be the ideal, simple and cost effective stain for assessement of overall sperm morphology in a rural tertiary care set-up. Haematoxylin stain was observed to be the best stain for assessement of sperm heads. However, a small margin has to be provided for inter-observer variations. Sperm morphology assessment remains the baseline necessity for the diagnosis and management of male factor associated infertility when advanced techniques are unavailable, inaccessible or unaffordable.