Invitro Study of the Effect of Different Samples of Water Used for Washing the Etchant on Bracket Bond Strength
Sandesh Phaphe1, Chanamallappa Ganiger2, Yusuf Ahammed3, Pratap Mane4
1 Associate Professor, Department of Orthodontics, School of Dental Sciences, KIMSDU, Karad, Satara, Maharashtra, India.
2 Associate Professor, Department of Orthodontics, School of Dental Sciences, KIMSDU, Karad, Satara, Maharashtra, India.
3 Senior Lecturer, Department of Orthodontics, School of Dental Sciences, KIMSDU, Karad, Satara, Maharashtra, India
4 Senior Lecturer, Department of Orthodontics, School of Dental Sciences, KIMSDU, Karad, Satara, Maharashtra, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Sandesh Phaphe, Associate Professor, Department of Orthodontics, School of Dental Sciences, KIMSDU, Karad, Dist: Satara, Maharashtra–415110, India. E-mail : sandeshphaphe2541@gmail.com
Background
Bonding is a very important step in the orthodontic treatment planning. Effective bonding enhances the treatment by reducing the bond failure and thereby reducing the treatment duration and also increases efficiency in orthodontic mechanics. The success of the bonded brackets is negatively affected by contamination with oral fluids such as blood and saliva.
Aim
The aim of the present study was to evaluate the effect of hardness of water used in removing the etchant on the bracket bond strength.
Materials and Methods
Seventy five extracted premolars were divided in three groups of 25 each. The teeth in all the three groups were etched with 35% phosphoric acid. The etchant in each of the group I, II and III was removed using distilled water (soft), corporation water (moderately hard) and hard water respectively. Stainless steel brackets were attached using light cure bonding agent (transbond XT, 3M UNITEK) and cured for 10sec with a light cure unit. The shear bond strength was evaluated by mechanical testing machine. Statistically significant differences were defined for p < 0.05.
Result
The results showed significant increase in bond strength in samples where in soft water was used for cleaning the etchant on the bonding surface.
Conclusion
Hardness of water used for washing the etchant affects the bracket bond strength. Shear bond strength of soft water is significantly increased compared to moderately hard and very hard water.
Bonding contamination, Bond failure, Hardness of water
Introduction
Bonding is the most sensitive procedure during orthodontic treatment. The presence of moisture in the oral cavity is the most common cause for bond failure. Contamination causes plugging of the porosities which are produced by acid etching and this reduces the surface energy. As the resin penetration is impaired it leads to poor mechanical interlocking [1]. The detrimental effect of moisture on orthodontic bonding could be due to induction of the plasticizing effect in the polymer network caused by water absorption. The latter creates hydrated zones at the polar monomer sites and the oxidation of pendant C=C bonds attached to the network, which liberate by-products such as formaldehyde so producing a plasticizing effect [2]. Bonding of brackets requires a completely dry field of operation and involves a series of technique sensitive steps, as contamination during this period leads to bond failure. The aim of this study was, therefore, to evaluate the role of mineral content (hardness) of the water used for washing the etchant on the bond strength of the brackets. The null hypothesis tested was that there are no significant differences in bond strength when different levels of hardness of water are used for washing the etched surface.
Materials and Methods
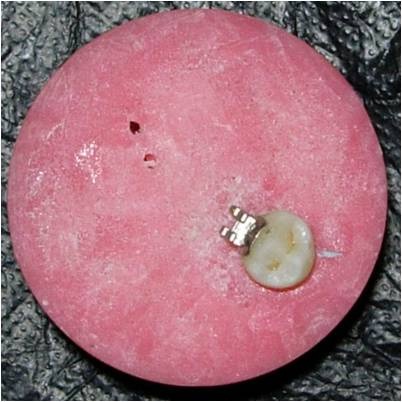
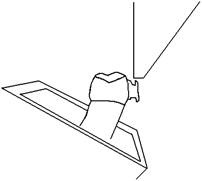
The present study was conducted in 2014 in the Department of Orthodontics, School of Dental Sciences, Krishna institute of medical sciences deemed university, karad. Seventy five human extracted premolars were collected which were extracted for orthodontic reasons. As per the selection criteria, the teeth had to be intact and free from restorations, caries, or previous surface treatment by acid. The teeth were divided in three groups of 25 each and mounted in an acrylic mould [Table/Fig-1] with cold cure acrylic. Non fluoridated prophylactic paste and rubber cups were used to clean the surfaces of all the teeth for 20 seconds to simulate recent cleaning [3]. The surface of each tooth was etched using 37% phosphoric acid (Transbond XT, 3M UNITEK). The etchant in group I was cleaned using distilled water (soft, 14.7ppm), group II with corporation water (moderately hard, 93.2ppm) and Group III with very hard water (216ppm) respectively. The hardness of water was measured with TDS machine (HM Digital, Inc). The samples of water were divided based on the hardness as classified by the U.S. Department of Interior and the Water Quality Association [Table/Fig-2]. Later, orthodontic brackets (RothP/1st and 2nd premolar S/D- Slot 0.18"; monalisa, JJ orthodontics, kerala, India) were bonded to the flattest surface of each tooth. The bond strength was measured in an Instron universal testing machine [Table/Fig-3]. The machine cross head speed was set at 1.0 mm/min, the recording speed was maintained at 20 mm/min, and the scale was set at 50 kg [4]. The force needed to disrupt the orthodontic bracket bond was verified for each sample [Table/Fig-4].
Acrylic mould with tooth and bracket mounted
The samples are separated based on the Water hardness as classified by the U.S. Department of Interior and the Water Quality Association [5]
| Classification | Mg/l or ppm | Grains/gal |
|---|
| Soft | 0 – 17.1 | 0 – 1 |
| Slightly | 17.1 – 60 | 1 – 3.5 |
| Moderately hard | 60 – 120 | 3.5 – 7.0 |
| Hard | 120 – 180 | 7.0 – 10.5 |
| Very hard | 180 and above | 10.5 and above |
NOTE: Other organizations may use slightly different classifications.
Instron machine model no 1122 (Instron India Ltd)

Diagrammatic representation of tooth with bracket mounted to receive shear force by instron machine
Statistical Analysis
Data obtained were subjected to chi-square statistical analysis. Statistical significance was set at < 0.05.
Results
Shear bond strength are listed in [Table/Fig-5]. Shear bond strength for Group 1 (91.93 kgf/cm2) samples was significantly higher than values achieved by the other groups (p<0.05). No significant difference was observed for the mean bond strength between Groups 2 (90.89 kgf/cm2), and 3 (90.18 kgf/cm2).
Mean shear bond strength and standard deviation (SD) for each group (n= 25)
| Groups | Shear bond strength | Standard deviation |
|---|
| group I (Soft water) | 91.93 kgf/cm2 | ± 0.98 kgf/cm2 |
| Group II (Moderately hard water) | 90.89 kgf/cm2 | ± 0.76 kgf/cm2 |
| Group III (Very hard water) | 90.18 kgf/cm2 | ± 0.45 kgf/cm2 |
Discussion
There have been many studies which have shown the role of contaminants on the bracket bond strength but the role of hardness of water on bracket bond strength is still not established. The bond between the bracket and resin should be adequate to contradict the masticatory forces, the forces of arch wires and also for proper tooth movement in all three planes of space. The bond strength should also not cause damage to the enamel surface during bracket debonding. Numerous studies have suggested that, the adequate strength for clinical conditions ranges from 2.8 MPa to 10 MPa [6–8]. Water is described as soft, moderately hard and very hard based on the dissolved minerals, specifically calcium and magnesium. As the content of calcium and magnesium increases in water the hardness level of water increases, this is attributed to the increase in the multivalent cations dissolved in water [9]. The mineral content of the water varies with geographical variation. Water contamination has been shown to reduce bond strength [10] and even blood and saliva contamination reduces bond strength [11–13]. Although there is no health risk with hard water, the mineral build up on the fixtures and the tubing of dental water unit causes nuisance, as it is difficult to clean the clogging caused by mineral build up using soaps and detergent. The hardness of water can be controlled by packaged water softeners. They are divided as: precipitating and non-precipitating [14].
Precipitating water softeners include washing soda, Zeolite clays and borax. When these products react with calcium and magnesium ions they form an insoluble precipitate. Once the precipitate is formed these ions cannot react but they form precipitate which makes the water cloudy and leads to build up on water channels. These kinds of softeners increase the alkalinity of water and can lead to damage of skin upon exposure.
Non-precipitating water softeners use complex phosphates to sequester calcium and magnesium ions. These softeners do not increase the alkalinity and do not form the precipitate, these help in dissolving the soap curd for a longer period of time. Permanent installation of Mechanical water softening units helps to remove the calcium and magnesium ions and thereby help to maintain the plumbing systems [15]. Water softeners function on the ion exchange process. In this process, water is passed through a media bed containing sulfonated polystyrene beads. These beads are supersaturated with sodium. As the water passes through the softening material ion exchange takes place, the minerals attach to these resin beads and sodium is simultaneously released into water. After a period of time this resin bead becomes saturated with calcium and magnesium. The saturation is removed by recharging the resin bead by passing the salt solution (brine), which replaces the magnesium and calcium content from the waste water [16].
The present study showed that wherever precipitating water softeners are used there is greater possibility of bond failure. This could be due to ionic interference of the calcium and magnesium [5] with the etched surface once the etchant is washed and could also lead to clogging of the etchant enamel prisms. The particles of the precipitate embed within the enamel prism structures and are not dislodged during air drying. These particles become engaged with composite and can lead to loose mechanical interlocking thus reducing the bond strength.
The present study showed lesser bond strength in moderately hard and very hard water, as this could be due to interference of the increased mineral content of the water. The mineral content of the water precipitates the water channel. This while spraying can be released through the three way syringe and produce interference with the mechanical interlocking. This study showed that the use of contaminated water used for washing the etchant bond surface during bonding, reduces the strength of the brackets thus prolonging the treatment duration.
Saliva, moisture and blood are commonly studied for their influence on the bracket bond strength. Even the slightest of contamination leads to reduction in shear bond strength. In the present study the bond strength showed by contamination with hard water is 9.01 MPa which is higher compared to saliva contamination as showed by Bishara et al., [11] whereas EI-Kalla et al., studied the effect of Saliva contamination on bond strength of single-bottle adhesives to enamel and dentin and reported that saliva contamination showed no effect on the bond strength [11,17].
The bond strength of 8MPa showed by contamination with very hard water is similar to saliva contamination as reported by Compoy et al.,, their study showed higher bond strength on uncontaminated surface and the role of contamination before and after primer application showed no effect [18]. Bishara et al., studied the effect of disinfecting the water lines in the shear bond strength of orthodontic brackets and reported that iodizing the waterlines did not affect the bond strength [19].
Contamination with blood is the most common cause of bond failure especially during exposure of impacted canine Hobson et al., studied the effect of moisture and blood contamination on bond strength of a new orthodontic bonding material and showed significant increase in bond strength during dry bonding (15.6 MPa) when compared to, moist (12.8MPa) and blood contamination (11.6MPa) [20]. Prasad M et al., studied the Effect of moisture, saliva and blood contamination on the shear bond strength of brackets bonded with a conventional bonding system and self etched bonding system [21]. Their study showed that contamination reduces the shear bond strength of all the groups, in self etch bonding system, water and saliva had significantly higher bond strength compared to other groups.
The present study shows the importance of using softeners to remove the hardness of water as this could affect the bond strength of brackets, though microscopic evaluation of the etched surface after use of water spray is needed to correctly establish results of the present study.
Conclusion
The bond strength of bracket when using hard and moderately hard water is significantly lower compared with soft water. This is attributed to weak mechanical interference caused by the blockade of porosities by precipitated mineral crystals. There needs to be further studies to justify the results of the present study.
NOTE: Other organizations may use slightly different classifications.
[1]. Rajagopal R, Padmanabhan S, Gnanamani J, A comparison of shear bond strength and debonding characteristics of conventional, moisture-insensitive, and self-etching primers in vitroAngle Orthod 2004 74:264-68. [Google Scholar]
[2]. Eliades T, Katsavrias E, Eliades G, Moisture-insensitive adhesives: reactivity with water and bond strength to wet and saliva-contaminated enamelEur J Orthod 2002 24:35-42. [Google Scholar]
[3]. Vicente A, Bravo LA, Romero M, Ortíz AJ, Canteras M, Shear bond strength of orthodontic brackets bonded with self-etching primersAm J Dent 2005 18:256-60. [Google Scholar]
[4]. Hansen PA, Killoy W, Masterson K, Effect of brushing with sonic and counterotational toothbrushes on the bond strength of orthodontic bracketsAm J Orthod Dentofacial Orthop 1999 115(1):55-60. [Google Scholar]
[5]. Basiri A, Shakhssalim N, Khosdel AR, Pakmanesh H, Radfar MH, Drinking water composition and incidence of urinary calculus: introduction of new indexIran J Kidney Dis 2011 5(1):15-20. [Google Scholar]
[6]. Buonocore MG, Principles of adhesive retention and adhesive restorative materialsJ Am Dent Assoc 1963 67:382-91. [Google Scholar]
[7]. Vicente A, Toledano M, Bravo LA, Romeo A, De la Higuera B, Osorio R, Effect of water contamination on the shear bond strength of five orthodontic adhesivesMed Oral Patol Oral Cir Bucal 2010 15(5):820-26. [Google Scholar]
[8]. Reddy L, Marker VA, Ellis E, Bond strength for orthodontic brackets contaminated by blood: composite versus resin-modified glass ionomer cementsJ Oral Maxillofac Surg 2003 61:206-13. [Google Scholar]
[9]. Kanadhia KC, Ramavataram DV, Nilakhe SP, Patel S, A study of water hardness and the prevalence of hypomagnesaemia and hypocalcaemia in healthy subjects of Surat district (Gujarat)Magnes Res 2014 27(4):165-74. [Google Scholar]
[10]. Cacciafesta V, Jost-Brinkmann PG, Süssenberger U, Miethke RR, Effects of saliva and water contamination on the enamel shear bond strength of light-cured glass ionomer cementAm J Orthod Dentofacial Orthop 1998 113:402-07. [Google Scholar]
[11]. Bishara SE, Oonsombat C, Ajlouni R, Denehy G, The effect of saliva contamination on shear bond strength of orthodontic brackets when using a self-etch primerAngle Orthod 2002 72:554-57. [Google Scholar]
[12]. Fu B, Sun X, Qian W, Shen Y, Chen R, Hannig M, Evidence of chemical bonding to hydroxyapatite by phosphoric acid estersBiomaterials 2005 26:5104-10. [Google Scholar]
[13]. Cacciafesta V, Sfondrini MF, Scribante A, De Angelis M, Klersy C, Effect of blood contamination on shear bond strength of brackets bonded with a self-etching primer combined with a resin-modified glass ionomerAm J Orthod Dentofacial Orthop 2004 126:703-08. [Google Scholar]
[14]. Knezovic NJ, Memic M, Mabic M, Huremovic J, Mikulic L, Correlation between water hardness and cardiovascular diseases in mostar city, Bosnia and HerzegovinaJ Water Health 2014 12(4):817-23. [Google Scholar]
[15]. Zhang S, Wang D, Chen YC, Zhang XW, Chen GJ, Absorption of calcium ion from aqueous solution using Na (+)- conditioned clinoptilolite for hot water softeningHuan Jing Ke Xue 2015 36(2):744-50. [Google Scholar]
[16]. Entezari MH, Tahmasbi M, Water softening by combination of ultrasound and ion exchangeUltrason sonochem 2009 16(3):356-60. [Google Scholar]
[17]. El-Kalla IH, Garcia-Godoy F, Saliva contamination and bond strength of single-bottle adhesives to enamel and dentinAm J Dent 1997 10(2):83-7. [Google Scholar]
[18]. Campoy MD, Vicente A, Bravo LA, Effect of saliva contamination on the shear bond strength of orthodontic brackets bonded with a self-etching primerAngle Orthod 2005 75(5):865-69. [Google Scholar]
[19]. Bishara SE, Soliman M, Ajlouni R, Laffoon J, Warren JJ, Waterline disinfectant effect on the shear bond strength of orthodontic bracketsAngle Orthod 2005 75(6):1032-035. [Google Scholar]
[20]. Hobson RS, Ledvinka J, Meechan JG, The effect of moisture and blood contamination on bond strength of a new orthodontic bonding materialAm J Orthod Dentofacial Orthop 2001 120(1):54-7. [Google Scholar]
[21]. Prasad M, Mohamed S, Nayak K, Shetty SK, Talapaneni AK, Effect of moisture, saliva and blood contamination on the shear bond strength of brackets bonded with a conventional bonding system and self etched bonding systemJ Nat Sci Biol Med 2014 5(1):123-39. [Google Scholar]