Gas in Hepatic Portal Veins with Gastric Massive Dilatation and Pneumatosis in Acute Pancreatitis
Maulana M. Ansari1, Nadeem Mushtaq2, Vibhor Pateria3, Imtiyaz Ahmad4, Nitin Kulshreshtha5
1 Professor, Department of Surgery, J. N. Medical College, Aligarh Muslim University, Aligarh, UP, India.
2 Senior Resident, Department of Surgery, J. N. Medical College, Aligarh Muslim University, Aligarh, UP, India.
3 Resident, Department of Surgery, J. N. Medical College, Aligarh Muslim University, UP, Aligarh, India.
4 Resident, Department of Surgery, J. N. Medical College, Aligarh Muslim University, UP, Aligarh, India.
5 Resident, Department of Surgery, J. N. Medical College, Aligarh Muslim University, UP, Aligarh, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Maulana Mohammed Ansari, B-27 Silver Oak Avenue, Street No. 4 End, Dhorra Mafi, Aligarh, U.P-202002, India. E-mail : mma_amu@yahoo.com
Gas in portal veins is a rare phenomenon observed secondary to bowel ischaemia and necrosis. A young girl with history of pica ingestion presented with acute abdomen with huge distension. Investigation revealed air in hepatic portal veins, air within stomach wall, and massive distension of stomach secondary to acute pancreatitis. Successful conservative treatment confirmed the current concept that all cases of hepatic portal venous gas do not warrant immediate surgical intervention.
Bowel ischaemia, Gastric dilatation, Portal vein gas
Case Report
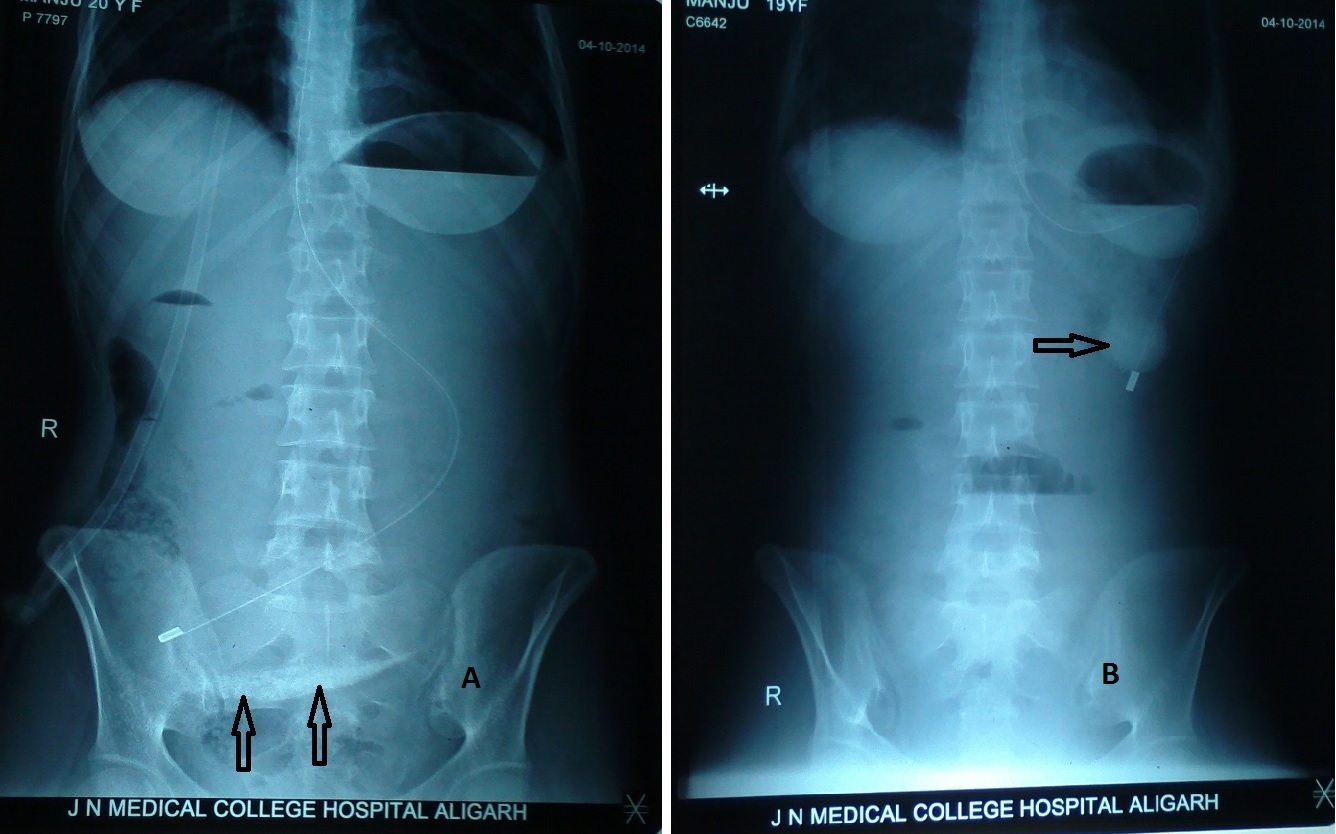
A 20-year-old girl presented to Jawaharlal Nehru Medical College Hospital Emergency Department with 1-day history of persistent diffuse abdominal pain, distension, constipation and one episode of vomiting. Vitals at presentation were pulse rate 128/min, BP 124/88 mmHg, respiratory rate 18/min, temperature 98.6o F. Cyanosis, icterus and paedal oedema were absent. Clinical examination revealed grossly distended abdomen with mild guarding and tenderness in the upper abdomen but there was no rebound tenderness. Liver dullness was present and there was no visible peristalsis. Bowel sounds were sluggish. On per rectal examination, soft faecal matter was present in the rectum and there was no rectal ballooning or tenderness. Intravenous fluid infusion was started and catheterization was done. Insertion of nasogastric tube (#16) resulted in siphon drainage of only 100 ml non-bilious faeculent aspirate with food particles that was followed by incomplete relief of abdominal distension and pain. Thereafter, abdominal X-rays (AXR) and ultrasound (USG) were done. Abdominal X-ray showed long length of the nasogastric tube inside abdomen, reaching upto the right iliac fossa [Table/Fig-1a], suggestive of huge gastric dilatation and ground glass appearance with a few innocuous looking gases in the right half of the abdomen, along with a suspicious radio-opacity overlying the sacrum. Suction drainage through the nasogastric tube resulted in 2 litres of non-bilious dirty aspirate containing food particles as well as certain non-food particles, which was followed by marked relief of the abdominal distension. The suspicious radio-opacity previously overlying the sacrum moved to the right upper quadrant after deflation of the stomach as seen on the repeat AXR [Table/Fig-1b]. USG of abdomen sowed bulky pancreas, echogenic material inside the stomach, and air within the wall of the stomach as well as air in the hepatic portion of the portal veins, and there were no gallstones. Unfortunately, USG film could not be made in the Emergency Department (ED). Next day Contrast Enhanced Computerized Tomography (CECT) with intravenous and oral contrast was performed that confirmed the diagnosis of the acute pancreatitis, air in the hepatic portal veins and air within the wall of the stomach; the stomach also contained significant amount of thick semi-split rather solidified material part of which was radio-opaque on non-contrast films [Table/Fig-2], although there was no previous history of contrast study in the recent past. Patient improved satisfactorily on continued conservative treatment. On 5th day, she developed one episode of fever and repeat Chest X-ray showed left-sided pleural effusion aspiration of which revealed 330 ml of straw-coloured fluid. Culture of pleural aspirate & blood was sterile. Patient started gradually increasing oral fluids and semisolids with normal bowel movements. On 10th day, patient developed generalized seizure that was controlled on intravenous infusion of Phenytoin sodium (20 mg/kg) and intravenous Prednisolone (2 mg/kg in two divided doses). Retrospectively, the patient had history of generalized seizure in childhood. Patient was discharged from the hospital on 12th day with advice for regular follow-up.
Abdominal X-ray: A – gross dilatation of stomach with nasogastric tube going into right iliac fossa and suspicious radio-opacity (arrows); B – suspicious radio-opacity moving to the right upper quadrant with change in shape (arrow)
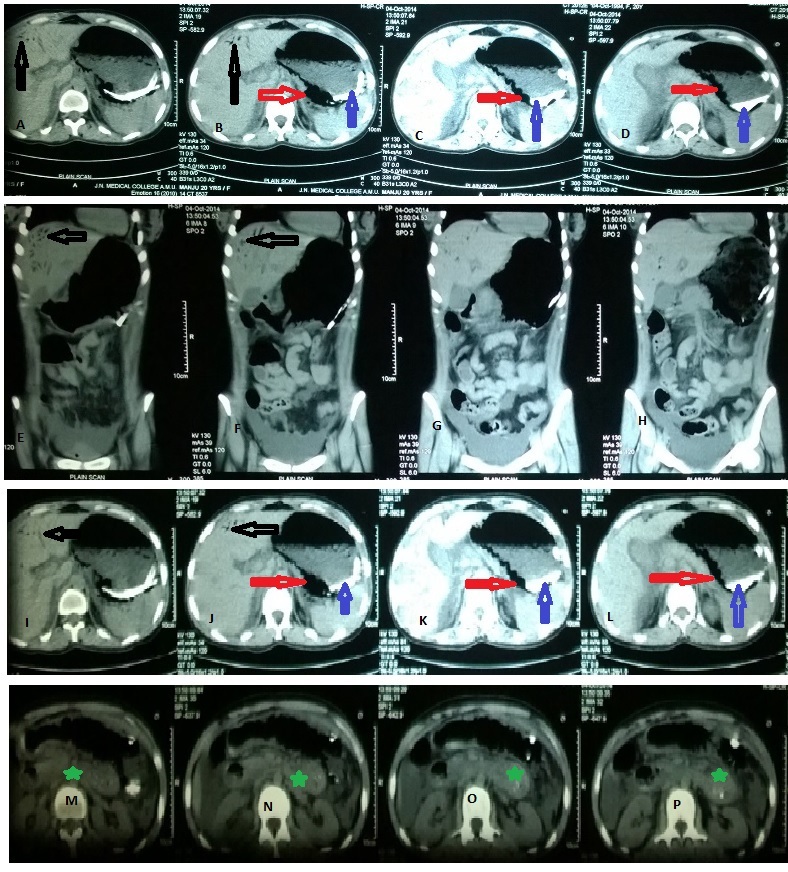
Computerized Tomography showing air in the hepatic portal veins (Black arrow), air in the stomach wall (Red arrow), radio-opaque material within the stomach (Blue arrow), acute pancreatitis (Green star)
Discussion
Gas in the hepatic portal veins (GHPV), a rare phenomenon, was first described in 1955 by Wolfe and Evans [1], and has been mostly described secondary to some form of bowel ischaemia and necrosis (in >75% of cases), but is also seen sometimes in other abdominal conditions and even after endoscopic procedures [2]. GHPV secondary to acute pancreatitis is extremely rare and seen by the senior most author first time in his career of over 30 years. Aetiopathogenesis of GHPV is largely unknown, and a number of hypotheses has been put forward [3,4]. Firstly, GHPV is microbe-derived gas production, secondly it is the absorbed intra-luminal air, thirdly it is the escape of gas into the portal circulation from increased pressure in the bowel and fourthly, it is the escape of gas into the portal circulation from increased pressure in an abscess cavity, fifthly it is due to presence of the gas-forming bacteria in the portal venous system [4–7]. Microbe-derived gases are said to be molecularly distinct from swallowed air [8]. Luminal air may enter the capillary veins either by an impaired epithelial barrier secondary to ischemic bowel and disrupted mucosa or by increased intra-luminal pressure [5,9], secondary to massive dilatation of stomach/bowel, especially in presence of pneumatosis or emphysema of the alimentary tract.
Presence of gas in the hepatic portal veins appears as alarming radiological finding in patients with acute abdominal pathology and is not regarded as a disease entity [3]. GHPV is usually detected on ultrasonography (USG) or CT scan, although it may sometimes be seen on conventional abdominal X-rays, but the peripheral branching linear radiolucencies in the right upper quadrant is often and easily overlooked [10,11], and the left lateral decubitus view may be more useful [1]. Radiologic tubular tree-like branching lucencies situated peripherally within 2 cm of the liver capsule and mostly transient are regarded as the GHPV while similar findings in the central portion of liver, i.e., near hilum and more than 2 cm away from the liver capsule are suggestive of the gas in the hepatic bile ducts and branching is usually fewer in number with lack of change in its pattern over several hours [12–14]. This distinction is possibly due to the difference of the slow centripetal flow of bile and the fast centrifugal flow of the portal blood [3]. Depending on the gas load delivered to the liver, the portal flow rate, and the patient’s posture, the antero-superior aspect of the left lobe (representing the most anti-dependent portion of the liver) is the most common site for gas accumulation [13]. Despite high inter-operator variability, the typical ultrasonographic findings of GHPV include: 1) echogenic particles/bubbles flowing within the portal vein; 2) poorly defined, echogenic foci/bubbles within the nondependent hepatic parenchyma [15–17].
Digital CT has higher sensitivity for the diagnosis of GHPV as compared to USG and plain radiography [18]. Increasing utilization of USG and CT not only helps in early detection of GHPV in the setting of severe illnesses [19,20], but also differentiate benign and non-life-threatening causes of GHPV not requiring surgical intervention such as gastric dilatation, gastric ulcer, ulcerative colitis, diverticulitis, pelvic abscess, necrotizing enterocolitis, intra-peritoneal tumour, Crohn’s disease, cholangitis, pancreatitis, complications of endoscopic procedures and others [9,12]. Until recently, presence of GHPV was regarded as a poor prognostic factor, but is now said to be really related to the underlying primary pathology [21].
In our patient, long length of naso-gastric tube within the abdomen seen on abdominal X-ray raised the suspicion of massive gastric dilatation but the subtle presence of air in the right upper quadrant and the gastric wall was realized only retrospectively. Pica material within the stomach, although radiologically evident in the initial X-rays, was also missed until USG was done. Prompt relief of the abdominal distension after the naso-gastric decompression and the markedly increased levels of serum amylase (577 U/L against reference normal value of 25-115 U/L) and lipase (2748 U/L against reference normal value of 73-393 U/L) diagnostic of acute fulminant pancreatitis guided us to continue the conservative treatment despite the high risk of impending perforation in presence of severe gastric emphysema and presence of unusual pica material within the stomach, although the first CT showed no evidence of pancreatitis and a diagnosis of the acute pancreatitis was made after verbal discussion with the radiologist. Aetiology of GHPV in our patient may possibly be ischaemic injury to the gastric mucosa already damaged by the chronic presence of the heavy pica material, secondary to the toxic massive gastric dilatation due to acute fulminant pancreatitis, which caused air to enter small mesenteric veins as suggested by Wu and Wang [2].
The present case supports the current concept that the indication for emergency abdominal exploration in hepatic portal vein gas should be based on the underlying primary aetiology rather than GHPV per se, and conservative treatment inclusive of broad-spectrum antibiotics is sufficient in majority of the cases, with only abdominal sepsis and intestinal necrosis warranting surgical intervention [11]. That is the reason why differential diagnosis of GHPV, in terms of the current best surgical practice, is very important because of its influence on patient management as the gas in the hepatic portal vein and the gas in the intra-hepatic bile ducts can appear similar radiologically [12]. Gas in the intra-hepatic bile ducts (Pneumobilia) suggests infection by gas-forming bacteria or an abnormal communication between the biliary tract and the intestines, and almost never merits consideration for emergency surgery; on the other hand, gas in the portal venous system may or may not be an ominous sign, because an urgent abdominal exploration is reserved only for presence of suspected/overt intestinal ischemia/necrosis and abdominal sepsis.
Conclusion
Present case of the gas in the hepatic portal vein, severe gastric pneumatosis and massive gastric dilatation reflects the vagary of clinical presentations of the acute fulminant pancreatitis, taxing the clinical acumen of the treating surgeon. Ultrasonography and contrast-enhanced CT is recommended for early differentiation of causal non-life-threatening conditions requiring the conservative approach from the life-threatening conditions warranting the immediate surgical intervention. Successful outcome following conservative treatment in our patient confirms the current concept that all cases of hepatic portal venous gas do not warrant immediate surgical intervention.
[1]. Wolfe JN, Evans WA, Gas in the portal veins of the liver in infants: a roentgenographic demonstration with postmortem anatomical correctionAm J Roentgenol Radiat Ther Nucl Med 1955 74:486-89. [Google Scholar]
[2]. Wu J, Wang M, Hepatic portal venous gas in necrotizing pancreatitisDig Surg 2009 26:119-20. [Google Scholar]
[3]. Allaparthi SB, Anand CP, Acute gastric dilatation: a transient cause of hepatic portal venous gas—case report and review of the literatureCase Rep Gastrointest Med 2013 2013:723160http://dx.doi.org/10.1155/2013/723160 [Google Scholar]
[4]. Nelson AL, Millington TM, Sahani D, Chung RT, Bauer C, Hertl M, Hepatic portal venous gas: the ABCs of managementArch Surg 2009 144(6):575-81. [Google Scholar]
[5]. Liebman PR, Patten MT, Manny J, Benfield JR, Hechtman HB, Hepatic-portal venous gas in adults: etiology, pathophysiology and clinical significanceAnn Surg 1978 187(3):281-87. [Google Scholar]
[6]. Kennedy J, Holt CL, Ricketts RR, The significance of portal vein gas in necrotizing enterocolitisAm Surg 1987 53(4):231-34. [Google Scholar]
[7]. Quirke TE, Hepatic-portal venous gas associated with ileusAm Surg 1995 61(12):1084-86. [Google Scholar]
[8]. Yale CE, Balish E, Wu JP, The bacterial etiology of pneumatosis cystoides intestinalisArch Surg 1974 109(1):89-94. [Google Scholar]
[9]. Kinoshita H, Shinozaki M, Tanimura H, Clinical features and management of hepatic portal venous gas: four case reports and cumulative review of the literatureArch Surg 2001 136(12):1410-14. [Google Scholar]
[10]. Gosink BB, Intrahepatic gas: differential diagnosisAm J Roentgenol 1981 137(4):763-67. [Google Scholar]
[11]. Kesarwani V, Ghelani DR, Reece G, Hepatic portal venous gas: A case report and review of literatureInd J Crit Care Med 2009 13(2):99-102.doi: 10.4103/0972-5229.56058 PMCID: PMC2772243 [Google Scholar]
[12]. Rajkovic Z, Papes D, Altarac S, Arslani N, Differential diagnosis and clinical relevance of Pneumobilia or portal vein gas on abdominal X-rayActa Clin Croat 2013 52:369-73. [Google Scholar]
[13]. Sebastià C, Quiroga S, Espin E, Boyé R, Alvarez-Castells A, Armengol M, Portomesenteric vein gas: pathologic mechanisms, CT findings, and prognosisRadiographics 2000 20(5):1213-26. [Google Scholar]
[14]. Faberman RS, Mayo-Smith WW, Outcome of 17 patients with portal venous gas detected by CTAm J Roentgenol 1997 169(6):1535-38. [Google Scholar]
[15]. Pan HB, Huang JS, Yang TL, Liang HL, Hepatic portal venous gas in ultrasonogram—benign or noxiousUltrasound Med Biol 2007 33(8):1179-83. [Google Scholar]
[16]. Lee CS, Kuo YC, Peng SM, Sonographic detection of hepatic portal venous gas associated with suppurative cholangitisJ Clin Ultrasound 1993 21(5):331-34. [Google Scholar]
[17]. Chezmar JL, Nelson RC, Bernardino ME, Portal venous gas after hepatic transplantation: sonographic detection and clinical significanceAm J Roentgenol 1989 153(6):1203-05. [Google Scholar]
[18]. Schulze CG, Blum U, Haag K, Hepatic portal venous gas imaging modalities and clinical significanceActa Radiologica 1995 36(4):377-80. [Google Scholar]
[19]. Gorospe EC, Benign hepatic portal venous gas in a critically ill patientSci World J 2008 8:951-52. [Google Scholar]
[20]. Hou SK, Chern CH, How CK, Chen JD, Wang LM, Lee CH, Hepatic portal venous gas: clinical significance of computed tomography findingsAm J Emerg Med 2004 22(3):214-18. [Google Scholar]
[21]. Monneuse O, Pilleul F, Barth X, Portal venous gas detected on computed tomography in emergency situations: surgery is still necessaryWorld Journal of Surgery 2007 31(5):1065-71. [Google Scholar]