Keratoacanthoma of Upper Lip: Review and Report of Case Managed Surgically
Saakshi Gulati1, Deepak Pandiar2, Selvan Kakky3, Achal Y Jiwane4, Anita Balan5
1 Senior Lecturer, Department of Oral Medicine and Radiology, Purvanchal Institute of Dental Sciences, GIDA, Gorakhpur, UP, India.
2 PhD Scholar, Department of Dentistry, Faculty of Dental Sciences IMS, BHU, Varanasi, UP, India.
3 Ex- Resident, Department of Oral Medicine and Diagnosis, GDCCalicut, Kerala, India.
4 Senior Resident, Department of Oral Medicine and Diagnosis, GDCCalicut, Kerala, India.
5 HOD, Department of Oral Medicine and Diagnosis, GDCCalicut, Kerala, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Deepak Pandiar, PhD Scholar, Faculty of Dental Sciences IMS, BHU, Varanasi, India. E-mail : deepakpandiar1923@yahoo.com
Keratoacanthoma is a benign lesion usually presenting as a solitary, dome shaped nodule with a central crater filled with keratin. It frequently occurs on the sun exposed areas of the skin. Keratoacanthoma can be difficult to differentiate from oral squamous cell carcinoma both clinically and microscopically. A case of keratoacanthoma involving the upper lip in a 51-year-old male is reported presenting as an exophytic growth that resolved after excisional biopsy.
Crater, Oral squamous cell carcinoma, Stratified squamous epithelium
Case Report
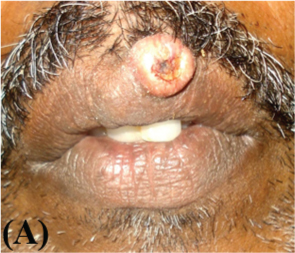
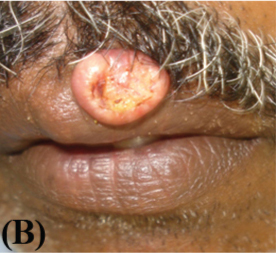
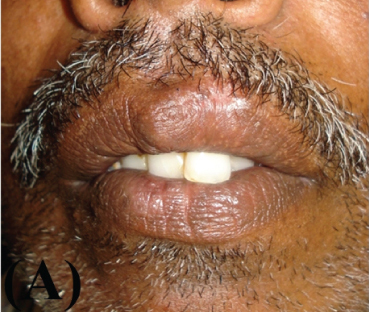
A 51-year-old male reported in the Department of Oral Medicine and Radiology, Government Dental College, Kozhikode, Kerala with the chief complaint of a growth over his upper lip of two weeks duration. His medical history included well controlled hypertension and asthma. There was no history of any deleterious habits. On examination, a raised well demarcated exophytic nodule was seen at the muco-cutaneous junction of the upper lip, about one cm from midline to the left side [Table/Fig-1a]. On palpation it was mildly tender, round, smooth, elevated, and sessile with well-circumscribed borders. The surface was erythematous and central portion had a shallow ulcer-like crater with brown and adherent encrustation. It was firm in consistency with no pus discharge. There was no history of trauma or paraesthesia associated with the swelling. An initial differential diagnosis of actinic cheilosis, keratoacanthoma, oral squamous cell carcinoma, molluscum contagiosum was made. The patient was not willing for the biopsy. However he reported a week later when the lesion rapidly increased in size [Table/Fig-1b].
Clinical presentation at the first time of reporting
Clinical presentation after one week (Note the increase in size)
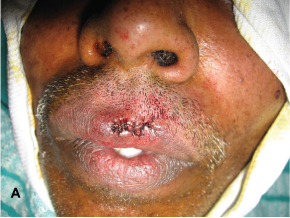
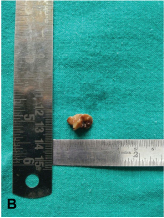
Subsequently the patient agreed upon the treatment and the lesion was completely excised in the Department of Oral and Maxillofacial Surgery and the tissue was sent for histopathological evaluation [Table/Fig-2a&b].
Immediate post operative photograph
Excised specimen after fixation in 10% formalin
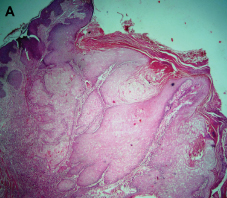
The histopathological section revealed a large crater surrounded by normal parakeratinized stratified squamous epithelium on both sides. The tip of the central crater formed an acute angle with the normal epithelium [Table/Fig-3a].
Photomicrograph of H&E section showing a large crateriform lesion with adjacent normal epithelium (40X)
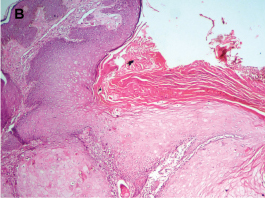
The crater showed hyper-ortho keratinized stratified squamous epithelium and epithelium at the base of the crater proliferating downward into the connective tissue showing no dysplasia [Table/Fig-3b&c]. Under high-power, the keratinocytes appeared enlarged with pale pink glassy cytoplasm [Table/Fig-3c]. The underlying fibrous cellular connective tissue was moderately infiltrated by chronic inflammatory cells [Table/Fig-3c]. Muscle bundles and lobules of sebaceous glands were also seen.
Photomicrograph of H&E section showing the crater filled with ortho-keratin (100X)
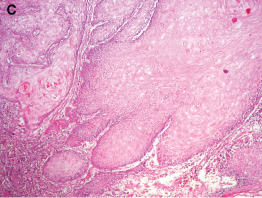
Photomicrograph of H&E section showing deeper portion of the lesion where it appears infiltrating the underlying connective tissue. Note that the individual cells do not show dysplastic features, keratinocytes shows glassy cytoplasm and the juxta lesional connective tissue is densely infiltrated by chronic inflammatory cells (100X)
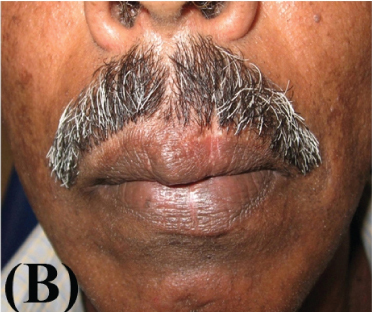
The patient reported seven days after the biopsy and favourable healing was observed. There was no sign of recurrence after a follow up of 6 months [Table/Fig-4a&b].
Clinical presentation at a follow up of one month;
Clinical presentation at a follow up of six months
Discussion
Keratoacanthoma (KA) is a rapidly growing lesion which masquerades histologically as oral squamous cell carcinoma (OSCC) [1]. But unlike KA, OSCC microscopically shows considerable cellular and nuclear anaplasia, pleomorphism with significant mitotic activity. Cribier et al., reviewed 296 cases and explained five histological criteria to differentiate KA from OSCC [2]. As in the present case, epithelial lip (the marginal buttress of the epithelium) and sharp outline between tumour and stroma were in favour of KA. Ulceration, numerous mitoses and marked pleomorphism/ anaplasia favoured OSCC in Cribier’s report [2].
The lesion appears to be fixed to the surrounding tissue and often grows rapidly where it is mistaken for OSCC. The difference in diagnosis between these two lesions is of uttermost significance since both entities have different management protocols. KAs are commonly found on the skin exposed to sunlight such as face, head and extremities. A frequency of 12% has been reported for solitary keratoacanthomas [3]. Upper lip is an uncommon site for the occurrence of keratoacanthoma, hereby we report a case of solitary keratoacanthoma of upper lip.
Schwartz considered KA as an aborted malignancy that only rarely progresses into an invasive squamous cell carcinoma [1]. The rapid evolution of KA is divided into three stages from an initial proliferative stage through fully developed lesion to involution. The lesion usually attains its maximum size in 4 to 12 weeks, after which it undergoes a slow process of involution extending over a period of two to four months. Occasionally, however, the lesions may persist up to 12 months. Although the KA usually appears as a solitary lesion, multiple tumours may be found and may be associated with various syndromes like Muir-Torre, xeroderma pigmentosum and nevus sebaceous of Jadassohn [4]. Other multiple lesion variants have also been described such as Ferguson Smith type and eruptive Grzybowski type [5].
The KA is likely to originate in the upper third of the hair follicle. Keratin production and growth cycle are two features of the follicular cells that can be recognized in the lesion. Thus, the growth of KA corresponds closely to the normal physiology of the hair follicle cycle. During the anagen phase a rapid growth occurs and destruction of tumour follicle epithelium results in regression of the tumour [6]. This is homologous to what happens in a hair follicle implying that an anagen hair follicle is destroyed when it changes from the anagen toward the telogen phase which may account for the benign behavior of keratoacanthomas. Recently, Wagner et al., also concluded in their immunohistochemical and histochemical study that KA of the lip is derived from upper outer root sheath cells [7]. In response to irritation or other stimulus, the hair follicle cells alter their normal growth cycle and produce a keratoacanthoma that imitates the growth cycle of the parent cells. Like most biologic phenomena however, no absolute law exists, and the growth may rarely become malignant [8,9]. Its aetiology is unclear, although exposure to ultraviolet light is the most common factor in the aetiology of KA’s [10]. This is supported by their usual occurrence on sun-exposed areas of the body, their increased incidence in subtropical areas. Other factors include exposure to tar, viruses, burns, oncogenic chemicals, epidermal growth factor and immunocompromised states. There are two schools of thoughts regarding the occurrence of KA. Some authors insist that KA’s do not occur on the vermilion of the lips [11]. They believed that KA’s arise from the pilo-sebaceous follicles of the skin and the latter are absent beneath the mucosa of the lip. However, many single cases of KA of the lip and oral mucosa have been reported in literature making it evident that KA’s do occur on the vermilion of the lip and that the presence of pilosebaceous follicles is not essential for their development [12,13].
Considering the common nature of KA’s and well differentiated OSCC and the lack of any features predicting prognosis, surgical excision of KA is advisable. The diagnosis can be made on the short history, with a plateau of growth and usually some definite resolution/involution noted by the patient and a central keratin plug and signs of fragmentation. The experience of the clinician is crucial. A striking clinical characteristic of KA is its rapid development. The lesion usually attains its maximum size in 4 to 12 weeks, after which it undergoes a slow process of involution extending over a period of two to four months. Occasionally, however, the lesions may persist up to 12 months. Other proposed treatments such as cryotherapy, electrodessication, irradiation, 5 FU (5-fluorouracil), curettage, x-ray, radium implantation, electrocautery, silver nitrate cautery, hydrocortisone and antibiotic ointments and podophyllin but the efficacy of these therapies is unclear [1,14]. Farias et al., recently proposed the treatment of KA with topical photodynamic therapy with methylaminolevulinic acid (MAL) cream [15]. While treating four patients they found that after three sessions of PDT, the lesions completely disappeared. There was no evidence of recurrence and excellent cosmetic outcome was achieved after three years of follow-up.
However, we propose that complete excision is the treatment of choice as it allows histological study of the complete lesion and moreover the resulting scar is usually less disfiguring than the scar remaining from a spontaneously healing lesion. Griffiths proposed principles for “watch and wait” management [16]. However, we believe that all suspected lesions should be excised surgically since all lesions do not spontaneously heal and even if they do, residual scar and disfigurement always remains. Lesions that are clinically identified as possible KA should be excised and submitted for histopathological confirmation to make an absolute distinction of this lesion from OSCC or other aggressive entity.
Conclusion
Keratoacanthoma at times poses difficulty in differentiation from oral squamous cell carcinoma and other aggressive entities. A correct diagnosis can avoid needless radical surgery.
[1]. Schwartz RA, KeratoacanthomaJ Am Acad Dermatol 1994 30:1-19. [Google Scholar]
[2]. Cribier B, Asch P, Grosshans E, Differentiating squamous cell carcinoma from keratoacanthoma using histopathological criteria. Is it possible? A study of 296 casesDermatology 1999 199(3):208-12. [Google Scholar]
[3]. Patil PB, Rathor V, Venkatraman S, Saxena S, Kamarthi N, Solitary keratoacanthoma involving upper lip: a diagnostic dilemma - case report and a brief reviewJ Clin Exp Dent 2010 2(1):e34-37. [Google Scholar]
[4]. Schwartz RA, Keratoacanthoma: a clinico-pathologic enigmaDermatol Surg 2004 30:326-33. [Google Scholar]
[5]. Neville BW, Damm DD, Allen CM, Bouquot JE, Oral and Maxilofacial Pathology 2002 2nd edIndiaElsevier Publication:354-55. [Google Scholar]
[6]. Ramselaar CG, Ruitenberg EJ, Kruizinga W, Regression of induced keratoacanthomas in anagen (hair growth phase) skin grafts in miceCancer Res 1980 40:1668-73. [Google Scholar]
[7]. Wagner VP, Martins MD, Dillenburg CS, Meurer L, Castilho RM, Squarize CH, Histogenesis of keratoacanthoma: histochemical and immunohistochemical studyOral Surg Oral Med Oral Pathol Oral Radiol 2015 119:310-17. [Google Scholar]
[8]. Davies DG, Kerato-Acanthoma or Squamous Carcinoma?J Laryngol Otol 1969 83:333-47. [Google Scholar]
[9]. El-Domeiri A, The question of recurrence and malignant transformation in keratoacanthoma (molluscum sebaceum)J Egypt Med Assoc 1971 54:68-73. [Google Scholar]
[10]. Ghadially FN, Barton BW, Kerridge DF, The Aetiology of KeratoacanthomaCancer 1963 16:603-11. [Google Scholar]
[11]. Rabut R, Hewitt J, Multiple successive kerato-acanthomas; labial localizationBull Soc Fr Dermatol Syphiligr 1954 61:14-16. [Google Scholar]
[12]. Spier HW, Thies W, Aggregierte Keratoacanthome (Mollusca Pseudocarcinomatosa)Hautarzt 1956 7:206-09. [Google Scholar]
[13]. Whittle CH, Davis RA, Keratoacanthoma of lower-lip red marginLancet 1957 272:1019-20. [Google Scholar]
[14]. Karnauchow PN, A lesion simulating squamous carcinoma of the lip (keratoacanthoma)Can Med Assoc J 1958 78:346-48. [Google Scholar]
[15]. Farias MM, Hasson A, Navarrete C, Nicklas C, Garcia-Huidobro I, Gonzalez S, Efficacy of topical photodynamic therapy for keratoacanthomas: A case-series of four patientsIndian J Dermatol Venereol Leprol 2012 78:172-74. [Google Scholar]
[16]. Griffiths RW, Keratoacanthoma observedBr J Plast Surg 2004 57:485-501. [Google Scholar]