One of the important aspects of endodontic treatment is to ensure proper asepsis to prevent any external contamination of the root canal systems [1], as high speed drilling equipments are used in the clinics, they produce aerosols which are heavily contaminated with both air born and oral microorganisms [1,2]. Since microorganisms are the main aetiological factor of pulp and periapical pathosis, a clinician’s primary goal should be to ensure their complete elimination during an endodontic treatment. Attention should be given not only to the endogenous oral microflora but exogenous bacterial contamination as well [3]. Guttapercha (GP) since its introduction has been most widely accepted as a prime root canal filling material [4]. Resilon is a thermoplastic polycaprolactone based root canal filling material which has the potential to challenge gutta-percha because of its ability to form a “monoblock™ in the canal [5–7].
Although Gutta-percha and Resilon cones are manufactured under aseptic conditions, once they are removed from their sealed packages and exposed to the operating environment, it has been shown to be contaminated with microorganisms such as rods, cocci and yeasts [2]. Operator’s fingers represent one of the most common and direct source of contamination of obturating material [8]. It has also been verified by Gomes et al., that 100% of Gutta-percha cones manipulated with gloves showed microbial growth [9]. Owing to the thermoplastic characteristic of Gutta-percha cones, unlike dental instruments they cannot be sterilized by conventional process in which moist or dry heat is used, as this may cause alteration of its structure. Therefore a rapid chair side chemical disinfection becomes essential [8]. Several chemicals have been experimented for this purpose like hydrogen peroxide, chlorhexidine, glutaraldehyde, sodium hypochlorite, iodine, alcohol and even MTAD [10].
Sodium hypochlorite (NaOCl) as an endodontic solution, is most widely used as an irrigant and for decontamination of rubber dam and obturating cones, in concentrations ranging from 0.5% to 5.25% [11]. Among the various agents investigated, immersing gutta-percha points in NaOCl at 5.25% concentration for 1 minute has been considered as a good standard for its rapid disinfection [12].
Royal MJ et al., demonstrated that MTAD – mixture of tetracycline isomer, an acid and a detergent which has been introduced as a final irrigant for disinfection of root canal system, can be used for the rapid disinfection of Gutta-percha and Resilon cones [1]. Results of one invitro study showed MTAD to be more effective than 5.25% NaOCl [13]. Chlorhexidine (CHX) is a broad spectrum antimicrobial agent [14] which has been used in several studies for rapid disinfection in 2% concentration but with contradictory results [6]. No general agreement exists about the optimal time required for decontamination of Gutta-percha which ranges from 1 min to more than 10 minutes [15].
Calcium hydroxide is a widely used intra-canal medicament. There are some strains of microorganisms that are resistant to the use of this drug hence it has been used in association with Metronidazole (Flagyl) and Ciprofloxacin i.e. Calcium hydroxide Flagyl Ciprofloxacin CFC) as an intracanal medicament in an exvivo study [16]. Minimum Inhibitory Concentration (MIC) is that minimum concentration of an agent when no growth bacteria were recorded. For CFC it was 0.125 mg ml-1 according to an invitro study which concluded that the most effective agent that could eliminate all the bacteria was CFC [9]. Other studies have also been reported where a combination of metronidazole with ciprofloxacin along with other agents have been effective in endodontic treatment of primary teeth [17].
The present study was undertaken to evaluate the effectiveness of 5.25% NaOCl, biopure MTAD, 2% CHX and CFC for the rapid disinfection of Resilon and gutta-percha cones contaminated with Enterococcus Faecalis.
Materials and Methods
This study was initiated in January 2010 and conducted in the Department of Microbiology of Yenepoya Dental College, Mangalore, India after obtaining an approval from the research ethical committee of the institute - YUEC 309/28/11/11.
Seventy new Gutta-percha (Dentsply Maillefer, USA) and Resilon (Pentron clinical technologies LLC, Wallingford, CT) cones were randomly chosen and each sample was contaminated by immersing in a suspension of E.faecalis (ATCC 14506) (Medi Mark, Europe) 109cfu/ml for 5 minutes.
To prepare CFC; Ca(oH)2 (Prime Dental Products Pvt. Ltd, India), Ciprofloxacin (500mg-Cipla Limited, India) and Metronidazole (400 mg - Lancer Health Care, India) were mixed in 2:1:1 ratio respectively. This mixture was then diluted in Glycerin (Prime laboratories, India) to make a concentration of 0.125 mg ml-1 [9].
These samples were then disinfected with the experimental agents for 30 seconds, 1 minute and 5 minutes respectively [1].
Hence the experimental groups were formed as follows:
GROUP 1- 5.25% NaOCl (Reachem Laboratory Chemicals Pvt. Ltd, India).
GROUP 2- Biopure MTAD (Dentsply Tulsa dental specialties, Oklahoma, USA).
GROUP 3- 2% Chlorhexidine (Hexidine, ICPA Health Products Ltd. India).
GROUP 4- CFC.
GROUP 5- positive control.
GROUP 6- negative control.
Fifteen cones of Gutta-percha and Resilon were chosen for each group, under which 5 cones each (subgroups) were disinfected for 30 seconds, 1 minute and 5 minutes respectively. For positive control, 5 Gutta-percha and 5 Resilon cones were contaminated with E.faecalis and washed with distilled water (Bio lab diagnostics India private limited) for 1 minute. For negative control, 5 Gutta-percha and 5 Resilon cones were chosen which were not contaminated with E.faecalis and disinfected with 5.25% NaOCl for 1 minute.
After the exposure of all the samples with the disinfectants, they were allowed to air dry and then washed by dipping in sterile water 5 times. All samples were then incubated in test tubes containing Brain Heart Infusion broths (Himedia Laboratories Pvt. Ltd., India) at 370C for up to 7 days. At first, third and seven days, broths were visually checked for turbidity by holding the samples up to a light and comparing the turbidity with that of the un-innoculated medium, which was clear.
Samples from each experimental and control groups were randomly chosen and plated on Mc Conkeys agar plate, then incubated at 37oC for 24 hours and checked visually for growth. If any growth was evident a gram stain essay was performed to confirm that the microorganisms growing were E.faecalis. Colony count from each group was recorded.
Statistical Analysis
The observed data was subjected to the statistical analysis of variance One-way ANOVA followed by Tukey’s post-hoc test and independent t-test at the significance level of α = 0.05.
Results
The observations were drawn from the colony count noted on the Mac Conkey agar plate. [Table/Fig-1] shows the observations of the bacterial colony count for various groups listed above where “A™ demonstrates Resilon and “B™ demonstrates Gutta-percha. The plates were divided from group Ito group VI as follows:
Observation on Mc conkeys agar plate – cfu recorded
Group 1 : 5.25% NaOCl, Group 2 : Biopure MTAD, Group 3: 2% CHX, Group 4: CFC, group 5: positive control, group 6: negative control
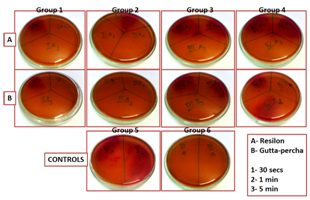
Group I- in which 5.25% NaOCl was used as a disinfectant,
Group II for MTAD,
Group III for 2% Chlorhexidine and
Group IV for CFC.
Group V and VI were the positive and the negative control respectively.
Each plate under each group was then subdivided into three sections using a marker to indicate the exposure time interval and was labeled as 1 for 30 seconds, 2 for 1 minute and finally 3 for 5 minute exposure in a clockwise direction. For E.g.: IA1 indicates Resilon cones disinfected using 5.25% NaOCl for 30 seconds.
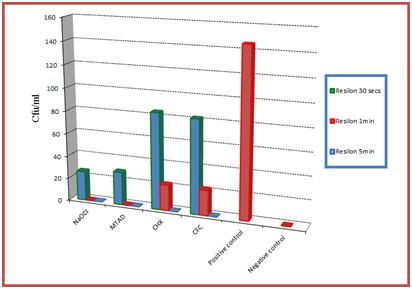
[Table/Fig-2,3] are the graphical illustrations of the obtained results for Resilon and Gutta-percha respectively.
Graphical representation of the results obtained for Resilon
Graphical representation of the results obtained for Gutta-percha
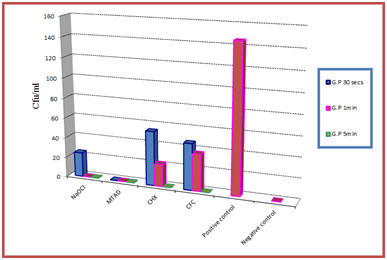
Group V (positive control) showed maximum E.faecalis growth, whereas with Group VI (negative control) no bacterial growth was recorded. According to results achieved all the agents evaluated were successful in eliminating E.faecalis from both Resilon and Gutta-percha, but the time of exposure required to disinfect all the samples differed. It was noted that Biopure MTAD (group II) was the only agent which could disinfect all gutta-percha cones after a 30 seconds exposure. However, it required 1 minute exposure for Resilon. 5.25% NaOCl (Group I) could effectively eliminate E.faecalis from both gutta-percha and Resilon cones after 1 minute of exposure. Two percent chlorhexidine (Group III) and CFC (group IV) both could not disinfect the samples with 1 minute of exposure and they required 5 minutes for effective disinfection.
Another observation made was that the cfu count of Gutta-percha for all the groups was comparatively less when compared with the cfu values of Resilon p < 0.05. For both Resilon and Guttapercha, at the end of 1 minute, the results obtained by Biopure MTAD and 5.25% NaOCl were significant when compared with 2% Chlorhexidine and CFC p < 0.05.
Discussion
Obturation of the root canal system is one of the most important steps to completely obliterate the root canal space. The contemporary concept of infection control requires every instrument or filling material that is placed within the root canal system to be sterilized [18]. Owing to the thermoplastic nature of the filling materials and the impossibility to determine the exact number of accessory cones required during obturation, an effective quick acting chemical agent is needed to prevent any possible contamination by microorganism [3].
Enterococcus faecalis (E.faecalis), anaerobic gram positive bacteria, has been consistently found in the ecosystem of persistant endodontic lesions [19], therefore, E.faecalis was used for this study as it is predominantly associated with failed RCT [20], and obturating with contaminated material can be one possible explanation for this phenomenon. Owing to its capacity to survive for long periods without nutrients, E.faecalis is usually associated with refractory lesions [16]. It also shows resistance to a wide range of antimicrobial agents [21], therefore if an agent was effective in eliminating E.faecalis, it would probably be effective against other microorganisms too. NaOCl and CHX are the two most commonly used endodontic irrigants which has found its use in several studies with various concentrations as a chemical disinfectant for obturating material [1,6,9,12,14,19,22].
Sodium hypochlorite acts as a powerful oxidizing agent by liberating active chlorine which inactivates the bacterial enzymes making it a powerful antibacterial and sporicidal agent [4]. In our study, 5.25% NaOCl when used for rapid disinfection, killed microorganisms in only 1 minute of exposure. This was in agreement with the results achieved by Sequeria et al., [15], Brenda Paula Figueiredo et al., [9] and Mathew et al., [1]. It was also demonstrated that 5.25% NaOCl eliminated even the spores from G.P after 1 minute of contact [15]. It was also reported that when used at a concentration of 5.25% NaOCl could effectively eliminate E.faecalis even 7 days post instrumentation [22].
Another agent evaluated was Biopure MTAD, which according to our study has shown success in eliminating E.faecalis. Its effectiveness is attributed to its anti-collagenase activity, low pH and its ability to be released gradually over time [23]. In this study MTAD required 30 seconds to disinfect Guttapercha cones and 1 minute for Resilon cones. Royal et al., had also achieved similar results [1]. According to another study MTAD was the most effective disinfectant (at 5 minute immersion) when compared with 5.25% NaOCl and 2% CHX, however the study also concluded that some amount of deposits were seen on the cones with all the agents used and that a final rinse with distilled water was essential to remove them [24]. Chlorhexidine acts as a bactericidal agent by disrupting the bacterial cell membrane and causing cytoplasmic precipitation [4]. In our study, 2% CHX was shown to be effective in disinfecting resilon cones only after 5 minutes of contact, this again was in agreement with the results achieved by Dumani A et al., and Zand V et al., which suggested that 1 minute treatment with 2% CHX was not sufficient to disinfect Resilon cones and that 5 minute exposure was required [25,6]. However, our results contradicted the findings achieved by Cardoso et al., and Gomes et al., who showed that 1 minute of treatment using 2% CHX was sufficient to disinfect gutta-percha or Resilon cones [26,9]. These contradictory results achieved even when same microorganism E.faecalis was used, suggests that more investigations need to be carried out [6].
Various studies have shown Ca(OH)2 to be ineffective against E.faecalis [27], hence it has been used in combination with Metronidazole and Ciprofloxacin to improve its antimicrobial activity. Metronidazole was the first choice because of its wide bacterial spectrum and effectiveness against oral obligate anaerobes [28]. However, some microorganisms may manifest resistance to metronidazole [14]. Hence an additional drug, Ciprofloxacin was mixed with calcium hydroxide and metronidazole in an attempt to eliminate all microorganisms. Ciprofloxacin acts by stopping the replication of the bacterial DNA and hence being bactericidal, and Metronidazole acts on anaerobic bacteria and parasites by showing selective toxicity. Pallota et al., concluded that CFC demonstrated the ability to eliminate all the bacterial strains at the lowest concentration used [16]. However, according to our study a 5 min exposure was mandatory to achieve disinfection.
Another observation of our study was that the colony count recorded for Gutta-percha samples were less when compared with Resilon. The primary components of gutta-percha are zinc oxide (75%) and Gutta-percha (20%). The remaining components are barium sulphate, resins and coloring agents [5]. The bactericidal effect of untreated G.P could be due to the relatively high content of ZnO besides other ingredients. Although barium sulphate is added as an X-ray contrast medium, it should be noted that it inadvertently exhibits a bacteriostatic or bactericidal effects depending on its concentration [2].
According to our study conducted 5.25% NaOCl and Biopure MTAD were the most effective agents for rapid disinfection of Gutta-percha and Resilon. However, since the duration of exposure was the only variable evaluated, other studies that are carried out to evaluate the surface free energy, wettability, alteration of the surfaces and physical characteristics etc also should be considered while deciding which agent is the most desirable. Even though most studies confirm the effectiveness of 5.25% NaOCl for rapid disinfection the main problem seems to be the changes noted in the structural and physical properties of the obturating materials which weren’t desirable [29] Prado et al., in an invitro study concluded that 2% CHX was the best disinfectant solution when compared with 5.25% NaOCl as it presented high values of surface free energy [30]. High surface energy results in better wettability therefore interfering positively with the adhesion mechanism. Valois et al., reported an increased elasticity of gutta-percha points after 1 minute immersion in 5.25% NaOCl [15], which can lead to difficulties in obturation especially in curved root canals [31]. Prado et al., verified the presence of chloride crystals when 5.25% NaOCl was used for disinfecting gutta-percha and Resilon cones [10], whereas, no topographic changes were observed with 2% CHX. The same study also showed that MTAD solution formed a layer that solidified and modified completely the topography of Gutta-percha, no such changes were seen with Resilon. It was hence concluded that final rinse with distilled water is essential mainly when NaOCl and MTAD are used for disinfection as they cause surface modifications which can interfere with the seal during obturation. CFC could be considered as a valid alternative considering the fact that along with being a good disinfectant, all the drugs are easily and routinely available in the dental operatory. Hence, though 2% CHX and CFC took longer time for disinfection of the samples, they still could be a better alternative considering the above mentioned findings which validates further studies. More over besides being an effective disinfectant, the agent should also be economical, routinely available in the clinics, consume minimal chair side time and most importantly cause minimal or no changes in the topography and properties of the obturating material.
Newer chemicals have been introduced for rapid disinfection with a promising future. Paracetic acid since its introduction have been indicated for high level disinfection, it was concluded according to a study that 1% Paracetic acid was effective in rapid disinfection of both Resilon and Gutta-percha [32]. According to a recent study which tested the efficacy of 3% sodium hypochlorite, 1% paracetic acid 2% chlorhexidine and 10% povidine iodine for the rapid disinfection of gutta-percha cones contaminated with Enterococcus faecalis and Bacillus Subtilis spores concluded that 1% paracetic acid and 2% chlorhexidine were the most effective Agents [29]. With the increasing trend towards the use of natural substitutes, agents like Aloe Vera which according to a study has shown to be effective in decontaminating gutta-percha cones within one minute, should be explored [4].
Conclusion
Within the limitations of the study, all agents could effectively eliminate E.faecalis from the infected Gutta-percha and Resilon cones. 5.25% NaOCl and Biopure MTAD took less chair side time i.e. one minute to effectively eliminate E.faecalis from all the samples when compared with 2% CHX and CFC which needed 5 minutes to achieve the same. However, since the duration of exposure was the only variable evaluated, other studies that are carried out to evaluate the surface free energy, wettability, alteration of the surfaces and physical characteristics etc. also should be considered while deciding which agent can be considered as the most effective one.