Optic strut and anterior clinoid process are surgically relevant bony landmarks in the parasellar region. Optic strut connects the body of sphenoid to the infero- medial aspect of base of anterior clinoid process. This is removed during anterior clinoidectomy and optic canal decompression. Superomedial margin of optic strut is significant in categorization of intradural and extradural aneurysms [1].
Knowledge of anatomical variations in the paraclinoid region like pneumatisation, carotico-clinoid foramen and inter- clinoid osseous bridge is essential in parasellar surgeries. Carotico-clinoid foramen is formed by the fusion of tips of anterior and middle clinoid processes. Inter-clinoid osseous bridge is formed by the fusion of anterior and posterior clinoid tips. Preoperative CT detection of carotico-clinoid foramen and inter-clinoid osseous ridge is required to prevent inappropriate retraction with consequent rupture of the cavernous segment of the internal carotid artery which results in fatal outcome [2]. With the advent of MDCT with multiplanar and 3D reconstructions, preoperative assessment of these anatomical variations is the norm [3].
The purpose of the present study was to evaluate the thickness, location and orientation of optic strut and anterior clinoid process in Indian population. Associated variations in the para-clinoid region were also studied. These parameters were studied solely based on MDCT images with multiplanar and 3D reconstruction.
Materials and Methods
The present study was a retrospective study evaluating essentially normal CT scans of head and paranasal sinuses of 95 patients of age group of 18-70 years. Institutional review board approval was obtained. Sample size of 95 patients was calculated based on an earlier study [3], with a precision of 10% at 95% confidence interval. CT sections were taken in a standardized axial plane in helical mode using a 16 detector scanner (GE Brightspeed Elite 16). Thin sections of 0.625 mm thickness were obtained with helical mode using 512x 512 matrix. Images in bone algorithm were processed for multiplanar and 3- dimensional reconstructions by NEC Multisync LCD 1990 SXi. No additional CT exposure/ change in radiation parameters were done for the purpose of the study.
A total of 202 consecutive scans of CT head and paranasal sinuses were reviewed, 90 cases were excluded due to presence of abnormalities, 12 were excluded due to presence of extensive internal carotid artery calcifications and 5 were excluded due to presence of beam hardening artefacts.
Beam- hardening artefacts, frequently visualized in the skull- base region and extensive wall calcifications involving cavernous and supraclinoid segments of ICA hinder visualization of parasellar region, especially in 3D reconstructed images. Hence, CT studies with beam hardening artefacts and cavernous and supraclinoid ICA calcifications were excluded from the study.
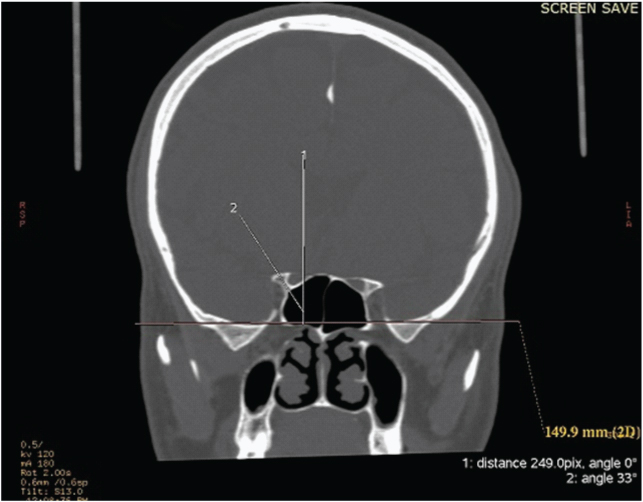
The thickness or the short diameter of optic strut and optic strut angle were measured using coronal reconstructed images in bone algorithm. The optic strut angle measured the inclination of the optic strut relative to the vertical in coronal reconstructed images [Table/Fig-1]. The 3D reconstructed images were aligned to provide an insight of the sella and parasellar region, optic canal, optic strut and anterior clinoid process.
Optic strut angle measurement – after aligning hard palate parallel to the horizontal, the angle is measured between the optic strut and the vertical
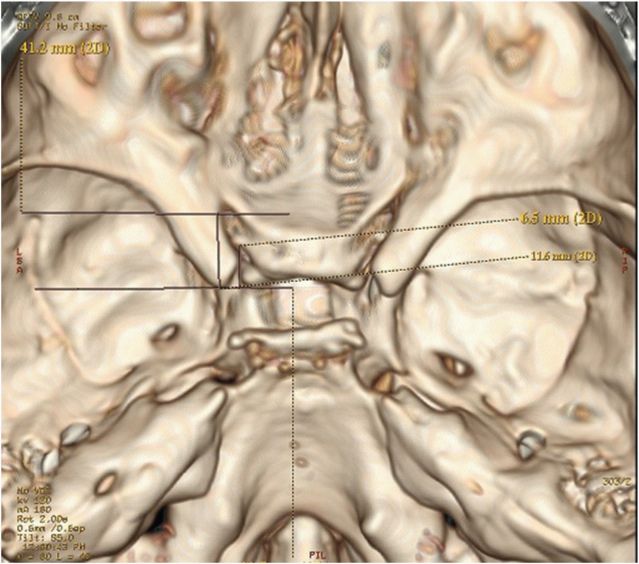
Using the 3D reconstructed images, the basal width of anterior clinoid process, the length of the anterior clinoid process (a) and the distance between the posterior margin of the optic strut to the anterior clinoid tip (d) were measured to assess the location of the optic strut at its attachment to the anterior clinoid process [Table/Fig-2]. The location of optic strut at its attachment to anterior clinoid process was calculated as a-d/a x100.
Optic strut angle measurement – after aligning hard palate parallel to the horizontal, the angle is measured between the optic strut and the vertical
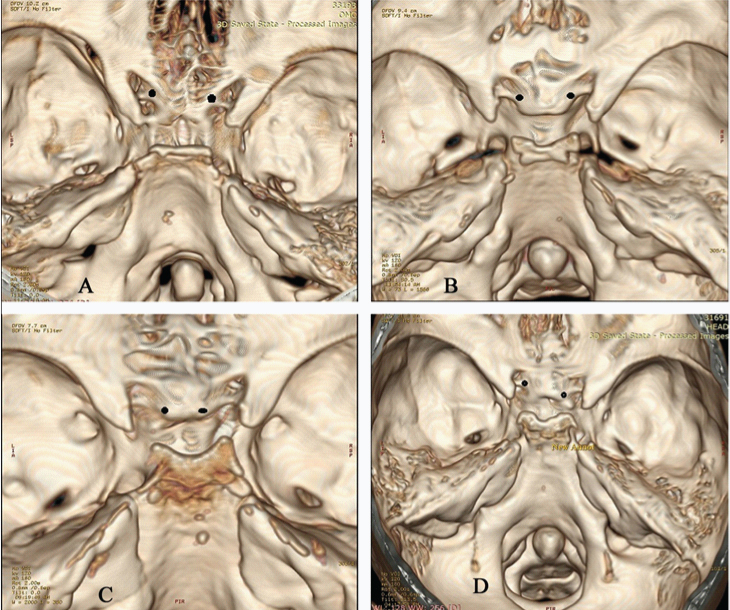
The attachment of the optic strut to the body of sphenoid was also assessed using 3D reconstructed images and were classified as sulcal, presulcal and post sulcal based on its location relative to prechiasmatic sulcus [Table/Fig-3]. The classification had been proposed in dry skull specimen in a previous study [3]. Prevalence of sulcal, presulcal and post sulcal attachments of optic strut to the body of sphenoid was calculated. Prevalence of carotico-clinoid foramen was assessed. Carotico-clinoid foramen was classified into complete, incomplete and contact types [Table/Fig-4]. Incomplete foramen showed a gap at the fusion of the anterior and middle clinoid processes, whereas contact type was described when the anterior and middle clinoid processes were connected by an inter-clinoid suture [4]. Other variations in paraclinoid region like optic strut pneumatisation and inter-clinoid osseous bridge were also studied [Table/Fig-5,6].
Location of optic strut relative to sphenoid body- Black dots represent the optic strut attachment
a) Presulcal type with the attachment is anterior to prechiasmatic sulcus. Note the additional presence of complete caroticoclinoid foramen on the left side
b) Sulcal type with the attachment to the anterior 2/3rd of prechiasmatic sulcus
c) Post-sulcal type with the attachment to the posterior 2/3rd of prechiasmatic sulcus
d) Asymmetric type- the attachment is postsulcal on the right side and sulcalon the left side
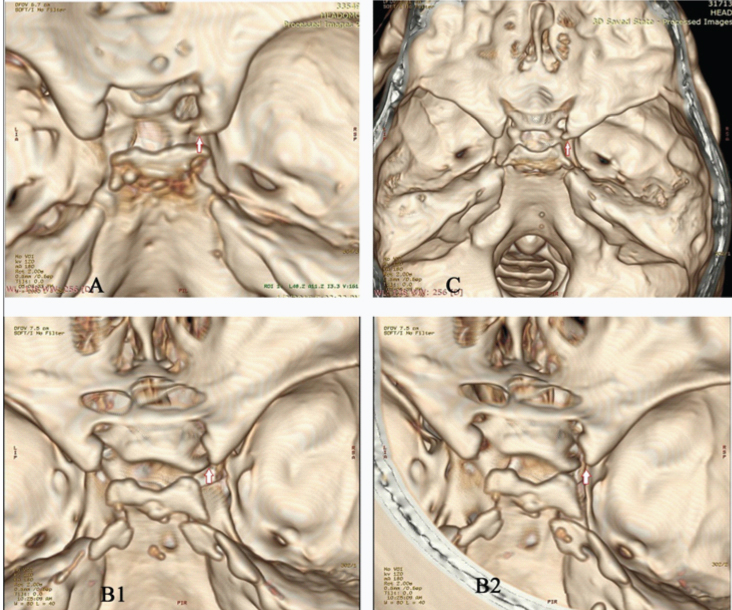
Types of carotico-clinoid foramina. Arrows (with red outline) indicate the carotico-clinoid foramina.
a) Complete carotico clinoid foramen (on right side)
b) B1- Incomplete carotico-clinoid foramen (on right side). B2- 3D reconstructed images has been tilted to demonstrate the gap between the anterior and middle clinoid processes.
c) Contact carotico-clinoid foramen (on right side)- fusion of anterior and middle clinoid processes with suture in between
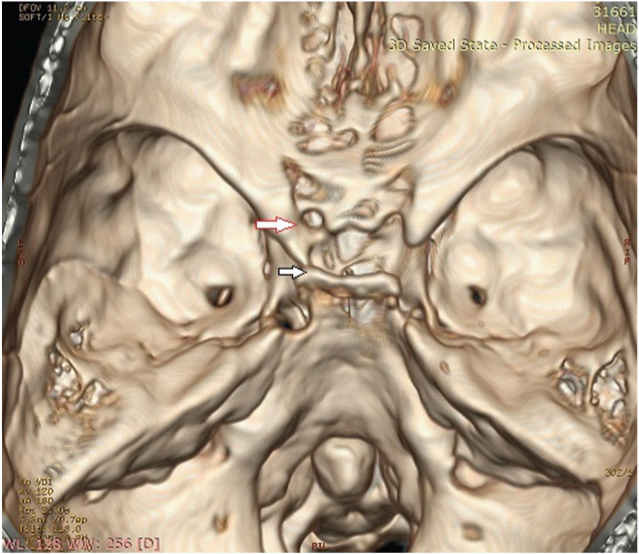
Inter-clinoid osseus bridge (on left side) fusion of anterior and posterior clinoid processes. Arrow (with red outline) indicates the inter-clinoid osseus bridge
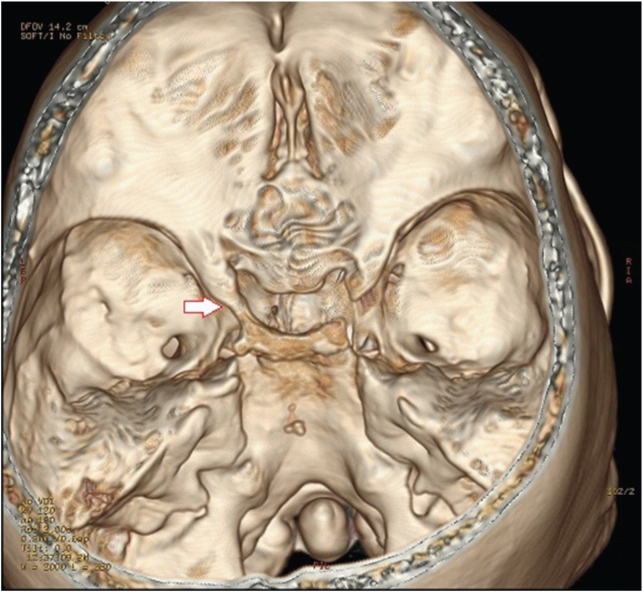
Presence of both complete carotico-clinoid foramen and inter-osseus bridge on left side. Arrow with red outline indicates carotico-clinoid foramen. Arrow with black outline indicates the inter-clinoid osseus bridge
Statistical Analysis
The data were analysed using SPSS15 for windows. The mean values of optic strut thickness, basal width and length of anterior clinoid process and optic strut angle was calculated and compared for statistical significance by independent t- test and one-way ANOVA test. p-value of 0.05 was considered significant.
Results
CT images of 95 subjects were studied which included 49 male and 46 female subjects. The age of these subjects ranged from 18 to 84 years (40.73 ±15.94 years).
The mean thickness of the optic strut was observed to be 3.64mm (±0.64mm). The thickness was larger on the right side (3.65mm±0.63) than the left side (3.62mm± 0.66). Thin optic strut of 2 mm was observed in 3 cases. The mean basal width and length of anterior clinoid process were measured to be 10.65mm (0.79mm) and 11.20mm (0.95mm) respectively. There was no statistically significant differences in dimensions of optic strut and anterior clinoid process between the analysed male and female subjects [Table/Fig-7]. Optic strut thickness in subjects of age- group 18-39 years was 3.56mm (0.64mm), among age group 40-59 years was 3.71mm (0.65mm) and among subjects of 60 years and above was 3.70mm (0.60mm). There was no statistically significant differences noted in the three age groups (p = 0.29).
Comparison of dimensions and orientation of optic strut and anterior clinoid process in males and females
| Mean (S.D) | p-value |
|---|
| Thickness ofoptic strut | Males (n=49) | 3.65(0.66) | 0.771 |
| Females (n=46) | 3.62(0.62) |
| Optic strut angle | Males (n=49) | 43.04(6.39) | 0.393 |
| Females (n=46) | 42.28(5.92) |
| Basal width of anteriorclinoid process | Males (n=49) | 10.65(0.76) | 0.977 |
| Females (n=46) | 10.65(0.82) |
| Length of anteriorclinoid process | Males (n=49) | 11.23(0.93) | 0.583 |
| Females (n=46) | 11.16(0.98) |
The optic strut angle showed a mean value of 42.67 degrees with a standard deviation of 6.16 degrees. Angulation was acute (<45 degrees) in 118 sides (62.11%) and flat (>45 degrees) in 72 sides (37.89%).
Optic strut attachment to the body of the sphenoid was classified into four types based on the location related to prechiasmatic sulcus. Presulcal attachment with medial margin located anterior to limbus sphenoidale was seen bilaterally in 05 cases (5.26%) of the reformatted CT images. Sulcal attachment was described when the medial margin was attached to anterior 2/3rd of prechiasmatic sulcus and was seen bilaterally in 52 cases (54.74%) of the reformatted CT images. Post sulcal attachment to posterior 2/3rd of sulcus was seen in 27 cases (28.42%) of the reformatted CT images. The position was asymmetrical in 11 cases (11.57%) of the cases. The location of the optic strut was also assessed using the 3D reconstructed image at its attachment to the anterior clinoid process. The most common location was observed to be at the anterior 2/5th of the anterior clinoid process (93 cases) and least was at the posterior 1/5th of the anterior clinoid process (4 cases). Attachment anterior to the base of the anterior clinoid process as described in previous studies was not seen in the present study [4,5] [Table/Fig-8].
Optic strut location relative to anterior clinoid process
| Location | Right (%) | Left (%) | Total Number(Percentage) |
|---|
| Anterior 1/5 | 4(4.21) | 2(2.10) | 6(3.16) |
| Anterior 2/5 | 44(46.32) | 49(51.58) | 93(48.95) |
| Anterior 3/5 | 39(41.05) | 32(33.68) | 71(37.37) |
| Anterior 4/5 | 6(6.31) | 10(10.53) | 16(8.42) |
| Posterior 1/5 | 2(2.10) | 2(2.11) | 4(2.11) |
The variation studied in this region included the presence of pneumatisation in the optic strut, presence of carotico-clinoid foramen and inter clinoid osseous ridge. Pneumatisation of the anterior clinoid process occurred in 28 sides with bilateral pneumatisation in 17 cases (17.89%) and optic strut pneumatisation in 23 sides. Carotico-clinoid foramen was observed in 42 cases (22.11%) and was classified into three types. A complete foramen was noted in 10 cases, an incomplete foramen in 24 cases and contact type was seen in 8 cases [Table/Fig-9]. Inter-clinoid osseous bridge is described when there is fusion of anterior and posterior clinoid processes and was seen unilaterally in 4 cases.
Distribution of carotico-clinoid foramen
| Type | Complete | Contact | Incomplete |
|---|
| Unilateral | Right | 5 | 1 | 09 |
| Left | 1 | 5 | 5 |
| Bilateral | 2 | 1 | 5 |
| Total, n (%) | 10 (5.26%) | 08 (12.63%). | 24 (4.21%) |
Discussion
The average thickness of the optic strut in this study measured 3.64 ± 0.64mm. Optic strut has been described as a wing shaped bone with thickness varying from 2.5 to 4 mm [6]. Kerr et al., measured the mean width of optic strut to be 4.23± 0.67 mm in American population [3] and Lee et al., measured it as 2.9±1.15mm in Korean population [5]. These differences were probably due to racial factors and different study methods.
The basal width and length of anterior clinoid process measured 10.65 ± 0.79 mm and 11.2 ± 0.95mm. The values are higher compared to the Korean study and similar to those of Nepalese population [5,7]. Study based on dry adult South Indian skulls has shown the average length and basal width of ACP on right side to be 10.68 mm and 12.4 mm respectively and on the left side to be 9.9 mm and, 11.12 mm respectively [8]. These variations could probably be due to different study methods and settings. Dimensions of the optic strut and ACP showed no significant difference between the genders, contradictory to an earlier study by Kapur et al., [9].
Wide ranges of optic strut angle ranging from 30.5 degrees to 57 degrees were recorded, which is in agreement to Kerr et al., and contradictory to description of relatively static angle (57 degrees) in study by Parkinson [3,6].
Previous studies have analysed the optic strut location relative to prechiasmatic sulcus and ACP in dry skulls [3,6,9]. In our study, we processed the 3-D volume rendered images and aligned them to visualize the central base of skull, including the optic strut and ACP.
Analysis of optic strut location relative to pre chiasmatic sulcus as suggested by Kerr et al., showed sulcal attachment to be the most frequent position, which is in accordance to the previous study [3]. Analysis of optic strut location relative to anterior clinoid process registered the attachment to anterior two fifth of ACP in 93 cases (48.93%) and was the most common position, which is consistent with the previous studies [5,9]. However, attachment anterior to the base of ACP, as described in previous studies was not seen in the present study [5,9].
Presence of pneumatisation, carotico-clinoid foramen and inter-clinoid osseous ridge are the anatomical variations in the paraclinoid region which could alter surgical outcomes [10]. Pneumatisation of ACP was seen in 28 sides (14.73%) which are similar to the results of previous studies (9.2% to 23.4%) [11,12].
Prevalence of carotico-clinoid foramen has been analysed in previous studies in dry skulls of various populations. Carotico-clinoid foramen was observed in 34 skulls (35.7%) and in 42 sides (22.11%) which are similar to studies by Erterk et al., and Inoue et al., [10,13]. However, unilateral osseous clinoid ridge was seen in 4 skulls (4.21 %) with results being close to Inoue et al and lower than Erterk et al., [10,13].
Anterior clinoidectomy is an essential step in entering cavernous sinus, in optic canal decompression and for a better surgical exposure in parasellar lesions like ophthalmic artery aneurysms, tumours in the anterior cavernous sinus and optic canal, neurovascular elements of sellar region and medial sphenoid wing. One of the complications of anterior clinoidectomy in pneumatised ACP is the opening of paranasal sinuses with risk of rhinorrhea. Cautious dissection would also be required with pneumatised ACP to avoid optic nerve and internal carotid artery injury. Carotico-clinoid foramen may affect the course of cavernous ICA and inter-clinoid osseous ridge may form a complete bony ring around clinoid ICA [14]. Also, complete removal of ACP in these cases would be difficult [10].
Extradural anterior clinoidectomy is an established and safe approach that allows extensive removal of the anterior clinoid process with creation of a wide operative field, especially in cases of para-clinoid aneurysm and tumours [15]. However, presence of carotico-clinoid foramen makes this approach difficult. Unsuitable manipulation in such cases would result in injury to internal carotid artery, optic and oculomotor nerves. To ensure a safe approach, combined extradural and intradural removal is recommended [16]. The carotico-clinoid foramen is covered by the distal dural ring, as seen intraoperatively and can be understood as a kind of bony distal dural ring [16].
Study by Stull et al., has confirmed the accuracy and reliability of CT 3D rendered volume images in autopsy remains [17]. Meticulous CT evaluation is vital in pre-operative assessment of para-clinoidal lesions. Optic strut and carotico-clinoid foramen have been previously studied in dry skulls with only few parameters like optic strut angle and pneumatisation being studied on MDCT images of dry skulls.
Limitations
The study has few limitations, basal width and length of anterior clinoid processes were measured on 3D volume rendered images and may not be accurate. However, assessment of optic strut attachment to the anterior clinoid process was measured as a ratio and hence would be acceptable. Optic strut dimensions and angle were studied in thin reformatted coronal images and hence would be accurate.
The study was based on unenhanced CT images. Therefore, lesions undetected on plain CT studies could not be excluded from the sample population. This could have included patients with intracranial aneurysm near the cavernous sinus. Also, anterior clinoid pneumatisation can affect the severity of beam hardening. Since studies with beam hardening artefacts were excluded, the prevalence of anterior clinoid pneumatisations could be altered. These are the other limitations of the study.
Conclusion
The present study is solely based on MDCT images of live subjects using the multiplanar and 3 dimensional reconstructions. 3D MDCT illustration of optic strut attachments to the sphenoid body and ACP base and 3D images of the various types of carotico-clinoid foramen, inter- clinoid osseus ridge are the nuances of this study. The study also attempts to provide morphometric features of clinoidal area and the prevalence of anatomical variations in South Indian population.
The study concludes that the mention of optic strut parameters, presence of carotico-clinoid foramen, inter-clinoid osseus bridge and pneumatisations, if any, is essential in parasellar lesions like paraclinoid aneurysms, tumours of the anterior cavernous sinus, medial sphenoid wing, and optic canal, if surgical approach has been considered. MRI, which the modality of choice in sellar and parasellar masses may not describe such bony variations in detail, especially in the presence of the pathology, therefore both CT and MRI are clinically relevant in these patients. Detailed preoperative evaluation with MDCT 3D and multiplanar reconstructions can provide prime information on the limited intraoperative view in paraclinoid lesions for a better surgical outcome.