Intraneural Hybrid Neurofibroma/Schwannoma In Scalp: A Case Report
Kishori Moni Panda1, Naik Reena2
1 Professor and HOD, Department of Pathology, Government Medical College (LSLAMMC), Raigarh, Chhattishgarh, India.
2 Demonstrator, Department of Pathology, Government Medical College (LSLAMMC), Raigarh, Chhattishgarh, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Kishori Moni Panda, Professor and HOD, Department of Pathology, Government Medical College, Raigarh, Chhattishgarh-496001, India. E-mail : drkishoripanda@gmail.com
Benign Peripheral Nerve Sheath Tumours (BPNSTs) are traditionally classified into schwannoma, neurofibroma and perinurioma. Due to advances in molecular techniques, hybrid BPNSTs containing more than one histologic types have been documented. Recent studies have demonstrated their frequent association with inherited syndromes like schwannomatosis and neurofibromatosis. Intraneural variant of hybrid neurofibroma/schwannoma is yet to be described. Here we report such a case in a 30-year-old male, who presented with a scalp swelling and histology showed intraneural neurofibromatous tumour admixed with schwannoma-like nodules. IHC (immunohistochemistry) showed variable S100 staining in neurofibromatous areas, diffuse S100 staining in schwannoma-like areas and negative EMA staining in the tumour.
Case Report
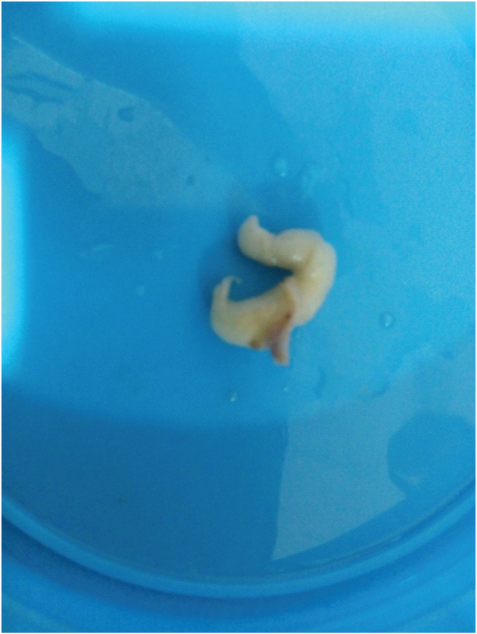
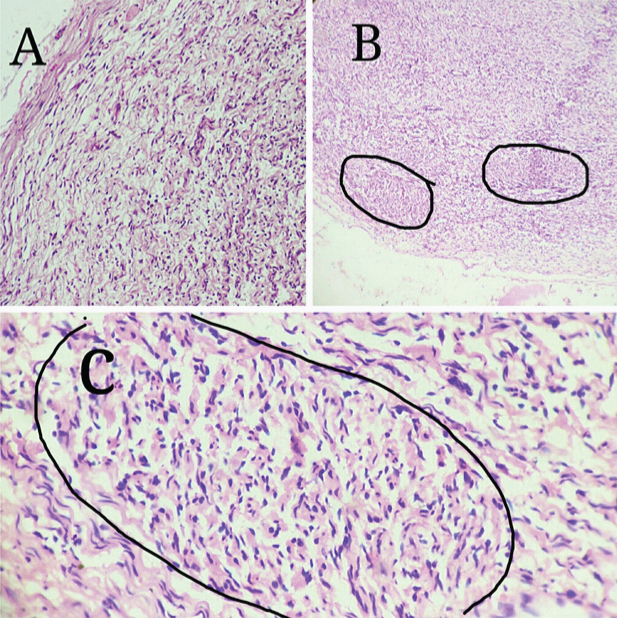
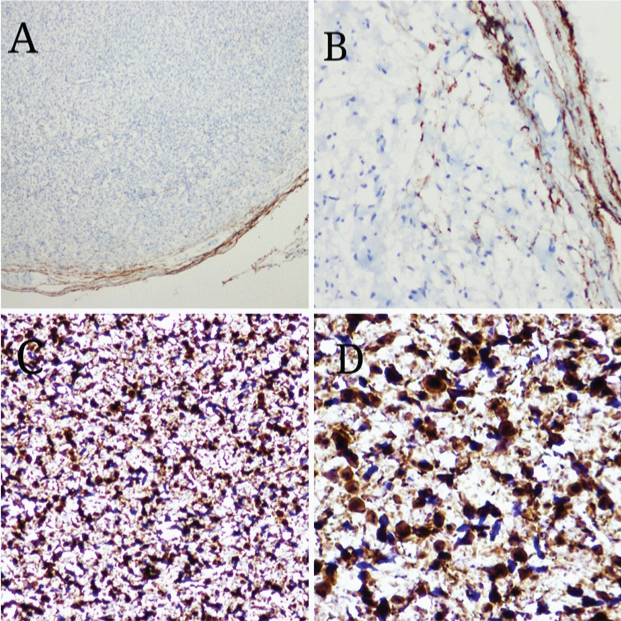
A 30-year-old male presented with swelling in the frontoparietal region of scalp of 2 months duration. On examination the mass was firm, mobile, elongated measuring about (4X1) cm. No stigmata of neurofibromatosis were found clinically. Provisional diagnosis was dermoid cyst and the mass was excised and sent for histopathological examination. Grossly, a fusiform mass measuring 4 cm in length and 1 cm in diameter, appearing like a thickened nerve received [Table/Fig-1]. Microscopy showed an intraneural biphasic tumour with intact perineurial sheath and had plexiform architecture due to presence of schwannian nodules [Table/Fig-2]. The predominant neurofibromatous component was composed of polymorphic population of cells like schwann cells with wavy serpentine nuclei, plump cells resembling fibroblasts and round to ovoid cells. The schwannoma-like part constituted multiple nodules composed of monomorphic schwann cells with wavy serpentine nuclei. Then IHC for S-100 & EMA was done, which showed strong and diffuse S-100 positivity in schwannian nodules in contrast to variable S-100 positive schwann cells admixed with other cells in neurofibroma-like component. Epithelial Membrane Antigen (EMA) staining was negative in the tumour cells and was focally positive in peripherally compressed perineurium [Table/Fig-3]. Combining histopathology and IHC findings it was reported as intraneural hybrid neurofibroma/schwannoma. On recent follow up after 6 months of excision, there was no recurrence and no other swelling has appeared in any other part of the patient’s body.
Gross showing fusiform enlargement of nerve,
a) Neurofibromatous component of the hybrid tumour with perineurial sheath (H&E,10X). b) Multiple schwannian nodules of the hybrid tumour (H&E, 10X). c) One of the schwannian nodules of the hybrid tumour (H&E, 40X),
a&b) IHC showing negative EMA staining in the tumour and focal positivity in the perineurium (10x & 40x). c&d) IHC showing variable S100 positivity in neurofibromatous area (10X & 40X)
Discussion
Hybrid BPNSTs are biphasic tumours showing discrete areas of more than one histologic types. For example: neurofibroma-schwannoma, schwannoma-perineurioma and neurofibroma-perineurioma. Hybrid neurofibroma/schwannoma are rare tumours that was first described by Feany and colleagues in a series of 9 cases, where they have noted the presence of a plexiform (multinodular) architecture in the majority of cases (five of nine) and they have interpreted these tumours as neurofibromas with schwannian nodules [1]. In other reported series, more frequent component of these benign hybrid tumours is perineurial. Michal M et al., has reported six tumours with hybrid schwannoma and perineurioma components [2]. Hornick JL et al., has also reported a subsequent series of 42 such cases [3]. Rare examples with hybrid neurofibroma and perineurioma features have also been reported [4,5]. Though intraneural variant of classic benign PNSTs is well known, intraneural variant of hybrid benign PNST is not described in the literature. Hybrid neurofibroma/schwannoma is frequently associated with schwannomatosis and neurofibromatosis syndromes [6,7]. The present case was not associated with any such syndromes.
Since the first report of hybrid BPNST in 1998 [1], a few cases have been described, with the great majority arising in peripheral nerves of the trunk and extremities. Uncommon locations, such as colon, nasal cavity, orbit, cranial nerve and lymph node, have also been reported [8–10]. Involvement of nerves in intraneural hybrid BPNSTs varies from inconspicuous to occasional presence of enlarged hypercellular nerves exhibiting plexiform involvement. Histologically, most of these tumours show an abrupt transition between the two components. In our case the transition between the two components is distinct and prominent.
The diagnosis of a hybrid BPNST is made in the presence of two distinct histological areas and is confirmed by different patterns of immunostaining of the two components. On IHC, schwannomatous component is diffusely S-100 protein positive (100%). But neurofibromatous component show variable S-100 protein positivity (30-70%) & CD34 positivity, whereas perineuriomatous areas are diffusely epithelial membrane antigen (EMA) positive [11]. In our case the tumour was intraneural causing fusiform expansion of the nerve, histology showed a plexiform architecture with admixture of two different components. In IHC, neurofibroma component showed variable S-100 protein-positive and -negative cells and schwannian nodules exhibited diffuse S-100 protein-positive cells.
Differential diagnosis of this case included localized intraneural neurofibroma, plexiform (intraneural nodular) schwannoma and intraneural perineurioma. Grossly intraneural neurofibroma may show typical fusiform expansion of the nerve trunk. But histology shows polymorphic population of cells i.e. schwann cells, perineurial cells, fibroblasts, mast cells, lymphocytes and CD34 positive spindle cells of unclear histogenesis. Our case differed from intraneural neurofibroma in having monomorphic schwannian nodules within polymorphic neurofibromatous tumour. Plexiform schwannoma occurs in superficial (cutaneous or subcutaneous) locations, shows plexiform monomorphic Antoni A pattern of growth and weakly (approximately 5% of cases) associated with schwannoma predisposition syndromes such as Neurofibromatosis and schwannomatosis [12,13]. In contrast our case showed admixture of both neurofibroma and schwannoma components. Intraneural perineurioma shows characteristic “pseudo-onion bulbs”. This feature was not seen in our case. So grossly, histologically and immunohistochemically our findings are consistent with those of hybrid neurofibroma/schwannoma, described in the literature.
Hybrid neurofibroma/schwannoma usually has a benign clinical course with only one recurrence documented. Malignant transformation in hybrid BPNST can be assumed to be extremely rare, though it is already documented [14]. The pathogenesis of the dual differentiation in hybrid neurofibroma and schwannoma may suggest that they may be histogenetically related than the earlier concept of two distinct separate tumours. However, Spinner RJ et al., have done genetic studies and have suggested that these hybrid tumours are two different clonal neoplasms, and is consistent with a collision tumour pattern [15]. Therefore origin of this hybrid tumour whether results from a localized micro environmental change or from a clonal genetic alteration remains to be further explored.
Conclusion
Hybrid morphologic features in BPNST may pose diagnostic problems, requiring advanced molecular diagnostic techniques. More molecular genetic studies are required to delineate its pathogenesis. Genetic screening in future may help in revealing its association with specific syndromes, which has prognostic and therapeutic implications.
[1]. Feany MB, Anthony DC, Fletcher CD, Nerve sheath tumours with hybrid features of neurofibroma and schwannoma: a conceptual challengeHistopathology 1998 32:405-10. [Google Scholar]
[2]. Michal M, Kazakov DV, Belousova I, Bisceglia M, Zamecnik M, Mukensnabl P, A benign neoplasm with histopathological features of both schwannoma and retiform perineurioma (benign schwannoma-perineurioma): a report of six cases of a distinctive soft tissue tumour with a predilection for the fingersVirchows Arch 2004 445:347-53. [Google Scholar]
[3]. Hornick JL, Bundock EA, Fletcher CD, Hybrid schwannoma/perineurioma: clinicopathologic analysis of 42 distinctive benign nerve sheath tumoursAm J Surg Pathol 2009 33:1554-61. [Google Scholar]
[4]. Kazakov DV, Pitha J, Sima R, Vanecek T, Shelekhova K, Mukensnabl P, Michal M, Hybrid peripheral nerve sheath tumours: Schwannoma-perineurioma and neurofibroma-perineurioma. A report of three cases in extradigital locationsAnn Diagn Pathol 2005 9:16-23. [Google Scholar]
[5]. Shelekhova KV, Danilova AB, Michal M, Kazakov DV, Hybrid neurofibroma-perineurioma: an additional example of an extradigital tumourAnn Diagn Pathol 2008 12:233-34. [Google Scholar]
[6]. Plotkin SR, Blakeley JO, Evans DG, Hanemann CO, Hulsebos TJ, Hunter-Schaedle K, Update from the 2011 International Schwannomatosis Workshop: from genetics to diagnostic criteriaAm J Med Genet A 2013 161:405-16. [Google Scholar]
[7]. Harder A, Wesemann M, Hagel C, Schittenhelm J, Fischer S, Tatagiba M, Hybrid neurofibroma/schwannoma is overrepresented among schwannomatosis and neurofibromatosis patientsAm J Surg Pathol 2012 36:702-09. [Google Scholar]
[8]. Emanuel P, Pertsemlidis DS, Gordon R, Yamada R, Mitsumaru A, Yokomizo H, Benign hybrid perineurioma-schwannoma in the colon. A case reportAnn Diagn Pathol 2006 10:367-70. [Google Scholar]
[9]. Kazakov DV, Pitha J, Sima R, Shelekhova K, Mukensnabl P, Michal M, Hybrid peripheral nerve sheath tumours: Schwannoma-perineurioma and neurofibroma-perineurioma. A report of three cases in extradigital locationsAnn Diagn Pathol 2005 9:16-23. [Google Scholar]
[10]. Youens KE, Woodward J, Wallace D, Cummings TJ, Hybrid neurofibroma-schwannoma of the orbitOrbit 2008 27:223-25. [Google Scholar]
[11]. Fletcher CDM, Peripheral neuroectodermal tumours. In: Fletcher CDM. (ed)Diagnostic Histopathology of Tumours 1996 New YorkChurchill Livingstone:1224-29. [Google Scholar]
[12]. Woodruff JM, Marshall ML, Godwin TA, Funkhouser JW, Thompson NJ, Erlandson RA, Plexiform (multinodular) schwannoma. A tumour simulating the plexiform neurofibromaAm J Surg Pathol 1983 7:691-97. [Google Scholar]
[13]. Fletcher CD, Davies SE, Benign plexiform (multinodular) schwannoma: a rare tumour unassociated with neurofibromatosisHistopathology 1986 10:971-80. [Google Scholar]
[14]. Rekhi B, Jambhekar NA, Malignant transformation in a hybrid schwannoma/perineurioma: Addition to the spectrum of a malignant peripheral nerve sheath tumourIndian J Pathol Microbiol 2011 54:825-28. [Google Scholar]
[15]. Spinner RJ, Scheithauer BW, Perry A, Amrami KK, Emnett R, Gutmann DH, Colocalized cellular schwannoma and plexiform neurofibroma in the absence of neurofibromatosis. Case reportJ Neurosurg 2007 107:435-39. [Google Scholar]