Radiotherapy is required as a primary treatment, as an adjunct to surgery, in combination with chemotherapy, or as palliation in majority of cases of invasive head and neck cancers [1]. Oral mucositis induced by radiation is defined as a reactive inflammatory like process of the oropharyngeal mucosa characterized by atrophy of epithelium, vascular damage, and an inflammatory infiltrate concentrated at the basement region. The epithelial atrophy is followed by ulceration [2–4]. The first signs of inflammatory changes in the mucosa are clinically seen at the end of the first week of a conventional radiation protocol. During radiation therapy, more severe stages of mucositis may develop, such as the formation of pseudomembranes (3 to 4 weeks) and ulcerations [5,6].
Mucositis is an inevitable but transient side-effect of radiotherapy causing serious complaints such as pain, swallowing, and speech problems [7]. Oral mucositis induced by chemotherapy is more acute than that due to radiotherapy [8]. The sites of predilection of mucositis induced by both radiotherapy and chemotherapy are the non-keratinized mucosa, such as the buccal and labial mucosa, the ventral and lateral surfaces of the tongue, the floor of the mouth, and the soft palate [9].
The current head and neck radiotherapy protocols have a mucositis incidence of 85-100% [10]. The severity of mucositis depends on different factors like cancer treatment protocol, age and diagnosis of the patient, level of oral hygiene during therapy, and genetic factors [11–13].
Acute phase proteins (APP) are a class of proteins whose concentrations fluctuate in response to inflammation. This response is called the acute phase reaction [14]. These proteins are produced by the liver and include C-reactive protein (CRP), serum amyloid A (SAA), serum amyloid P (SAP), fibrinogen and ferritin. APP contributes to host defense by directly neutralizing inflammatory agents, minimizing the extent of local tissue damage, as well as participating in tissue repair and regeneration [14]. The most studied of the APP is CRP, a protein that rises in the blood with inflammation. Increase in pro-inflammatory cytokines and acute phase proteins (APP) are associated with the development of mucositis and are likely to play important roles in mediating injury and in signaling pathways [15]. Most APP is induced between 50% and several folds over normal levels [14].
Scoring oral mucositis provides a means of evaluating the relationship between dosage, mode of delivery and complications. It is also useful in assessing the impact of therapy on patient morbidity, mortality and the quality of life of patients. The most commonly used scale is the World Health Organization (WHO) classification which grades oral mucositis into 0, 1, 2, 3 and 4 on the basis of clinical appearance and functional status [16].
Erythrocyte sedimentation rate (ESR) is an acute-phase reactant test that reacts to acute conditions in the body and is used to evaluate the health status of patients when an inflammatory, neoplastic or infectious disease is suspected [17].
This study is aimed at correlating CRP, ESR and total leukocyte count with radiation induced mucositis in patients receiving radiation treatment and concurrent chemotherapy.
Materials and Methods
The study was conducted in 2010 at the Department of Radiation Oncology, Father Muller’s Medical College and Hospital, Mangalore and A. B. Shetty Memorial Institute of Dental Sciences, Mangalore, India. The study comprised of 30 subjects each in the study group and control group. Patients in the study group were diagnosed with head and neck cancer and planned to undergo chemo-radiotherapy as the treatment option. The procedure involved clinical scoring, estimation of CRP, ESR and Total Leucocyte Count (TLC) in the study and control groups. These procedures were done on the first day prior to the commencement of radiotherapy. Subsequent samples were taken on the 7th, 14th, 28th and 42nd day. Only a single sample was collected from the control group which comprised of 30 subjects.
Oral mucositis was clinically evaluated and scored based on the WHO 1979 scale [18].
Grade 0: no change
Grade 1: Soreness / erythema
Grade 2: Erythema / ulcers / can eat solids
Grade 3: Ulcers / requires liquid diet only
Grade 4: Alimentation not possible
Statistical Analysis
Mann Whitney U-test and Chi-square test were used for group comparison and intergroup comparison respectively.
Results
Out of the 30 patients included in the study group, 25 were males and 5 were females. Thirteen patients received radiotherapy alone and 17 patients received chemo-radiotherapy. Carcinoma of the tongue constituted the major number of cases (14 cases) followed by carcinoma of oropharynx (3 cases), alveolus (3 cases), tonsil (2 cases), floor of mouth (2 cases), buccal mucosa (2 cases), supraglottis (2 cases), palate (1 case) and pyriform fossa (1 case).
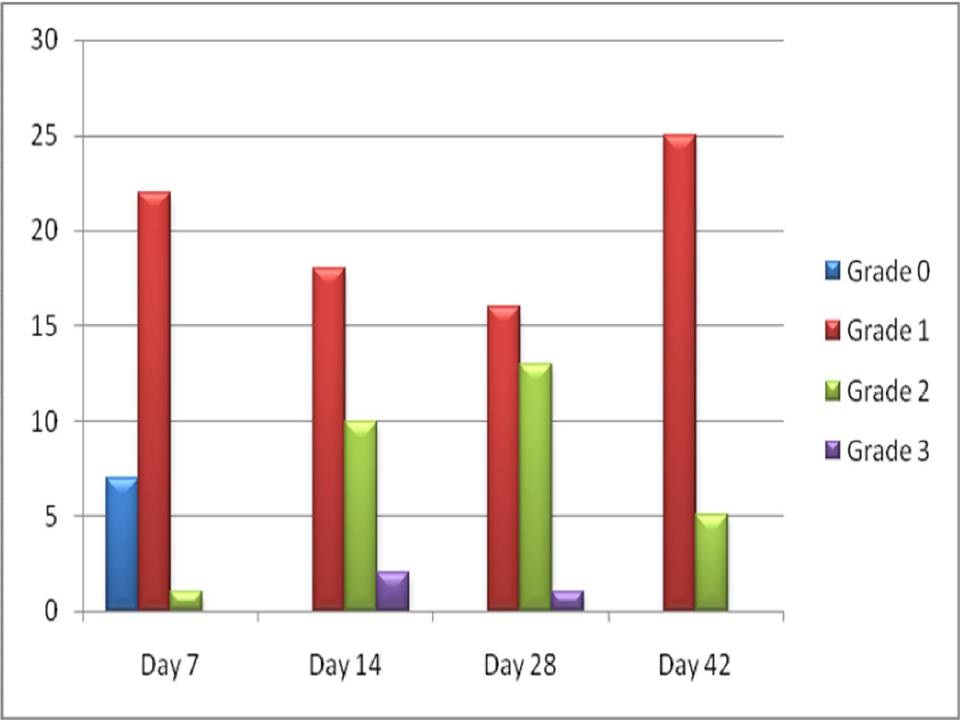
On day 7, 7 out of 30 patients did not develop any signs or symptoms of mucositis, 22 patients developed Grade 1 mucositis [Table/Fig-1] and only 1 patient developed Grade 2 mucositis. On day 14, 18 out of 30 patients continued with Grade 1 mucositis, 10 patients developed Grade 2 mucositis [Table/Fig-2] and remaining 2 patients developed Grade 3 mucositis [Table/Fig-3]. On day 28, 16 patients continued with Grade 1 mucositis, 13 patients developed Grade 2 mucositis and only 1 patient continued with Grade 3 mucositis. On day 42, 25 patients exhibited Grade 1 mucositis and the remaining 5 patients showed Grade 2 mucositis.
Patient with Grade I mucositis: soreness/erythema
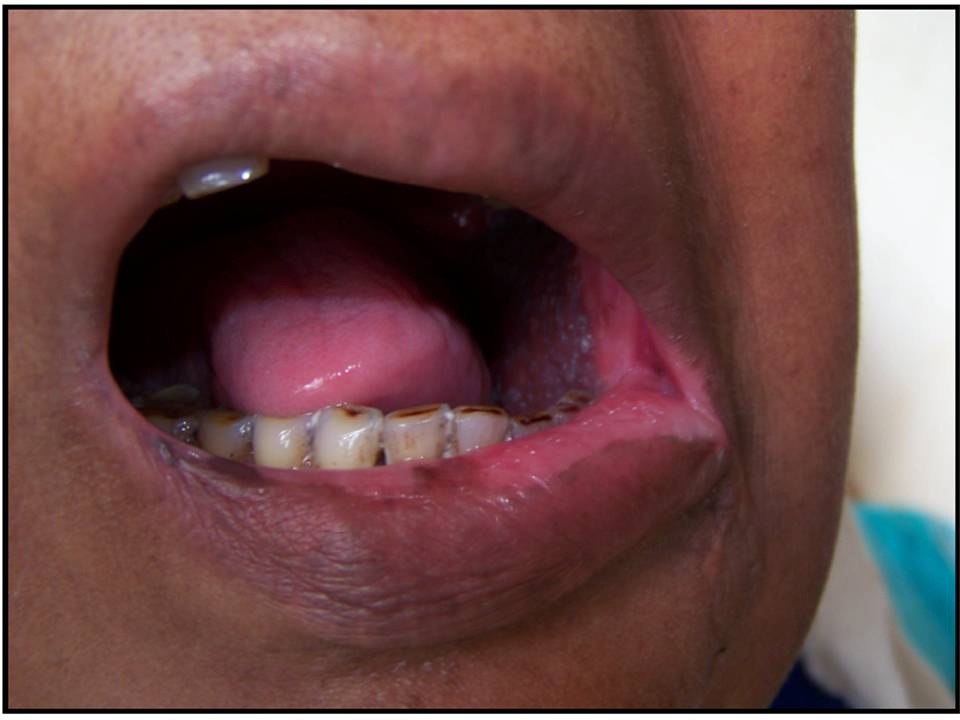
Patient with Grade II mucositis: erythema/ulcers/can eat solids
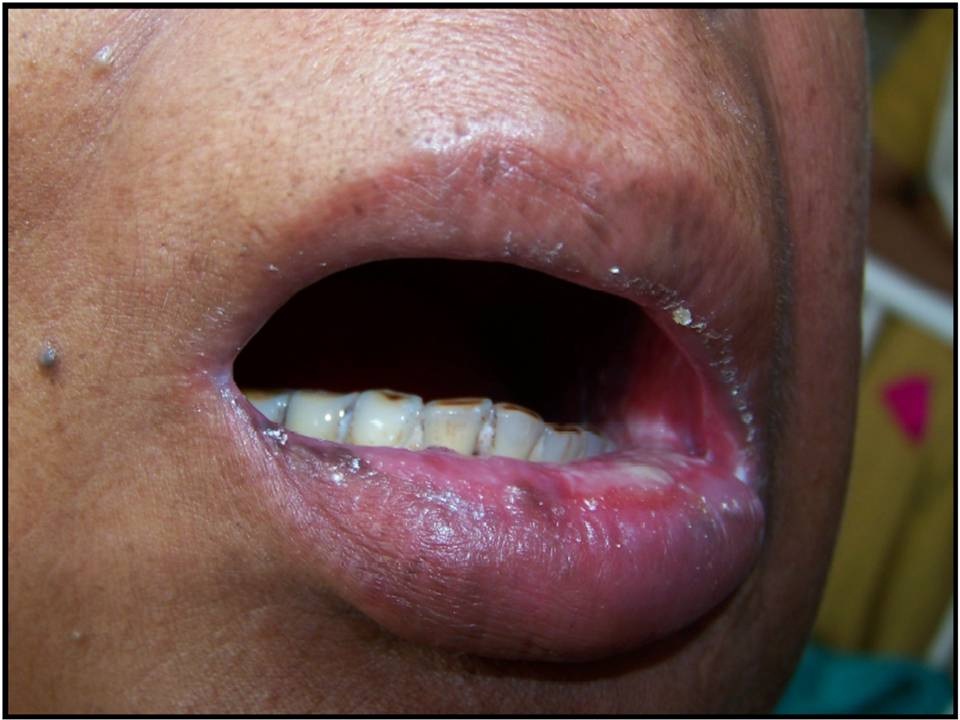
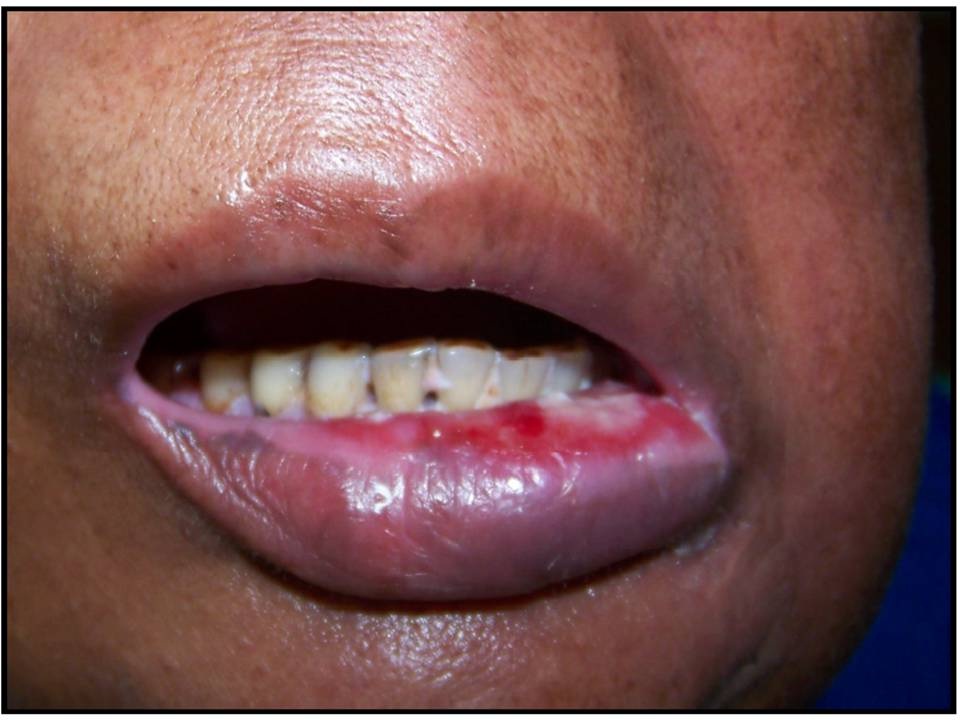
Patient with Grade III mucositis: ulcers/requires liquid diet only
Comparison of WHO mucositis grading from the beginning till the end of treatment protocol [Table/Fig-4] showed that there was a significant increase in the severity of mucositis during the course of treatment followed by a gradual decrease in severity towards the end of radiotherapy on the 42nd day.
Comparison of WHO mucositis
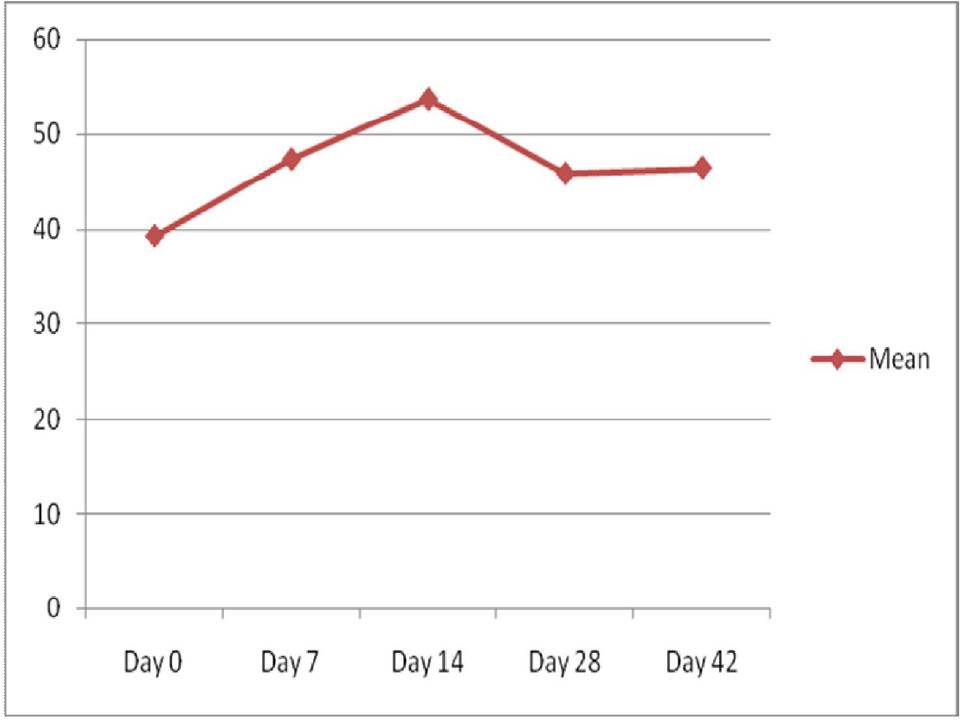
The comparison of mean CRP between the study group before the commencement of radiotherapy and the control group showed statistically a very highly significant difference. The comparison of CRP levels from day 0 to day 42 in the study group showed a significant increase towards the end of radiotherapy [Table/Fig-5].
Comparison of CRP levels from day 0 to day 42
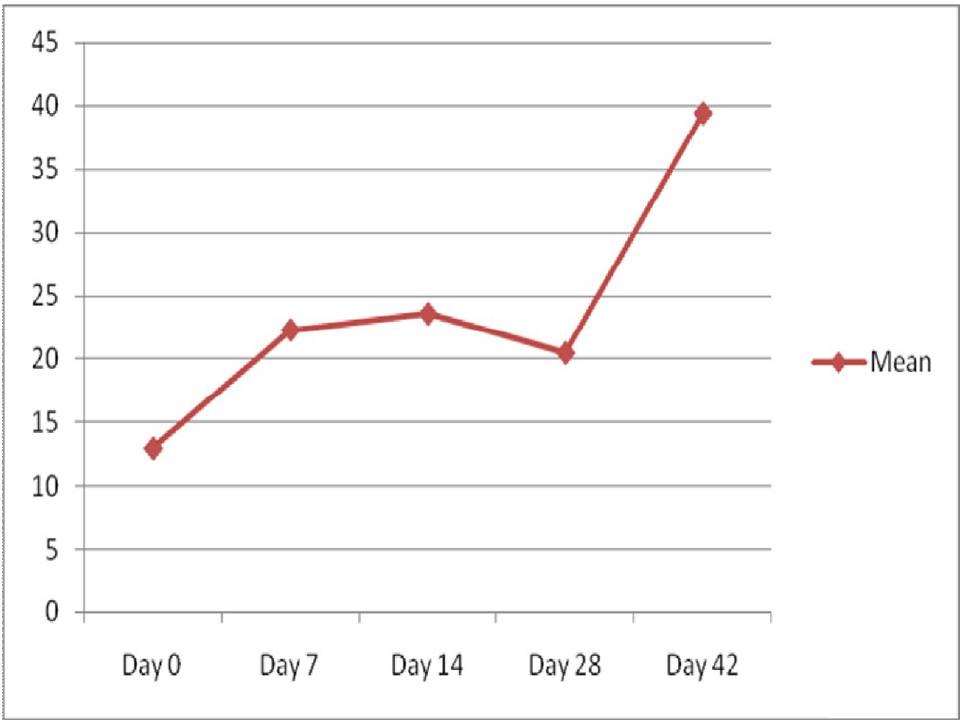
The comparison of mean ESR between the study group before the commencement of radiotherapy and the control group showed statistically a very highly significant difference. The comparison of ESR levels from day 0 to day 42 in the study group showed a significant increase till day 14 followed by a decrease towards the end of radiotherapy [Table/Fig-6].
Comparison of ESR levels from day 0 to day 42
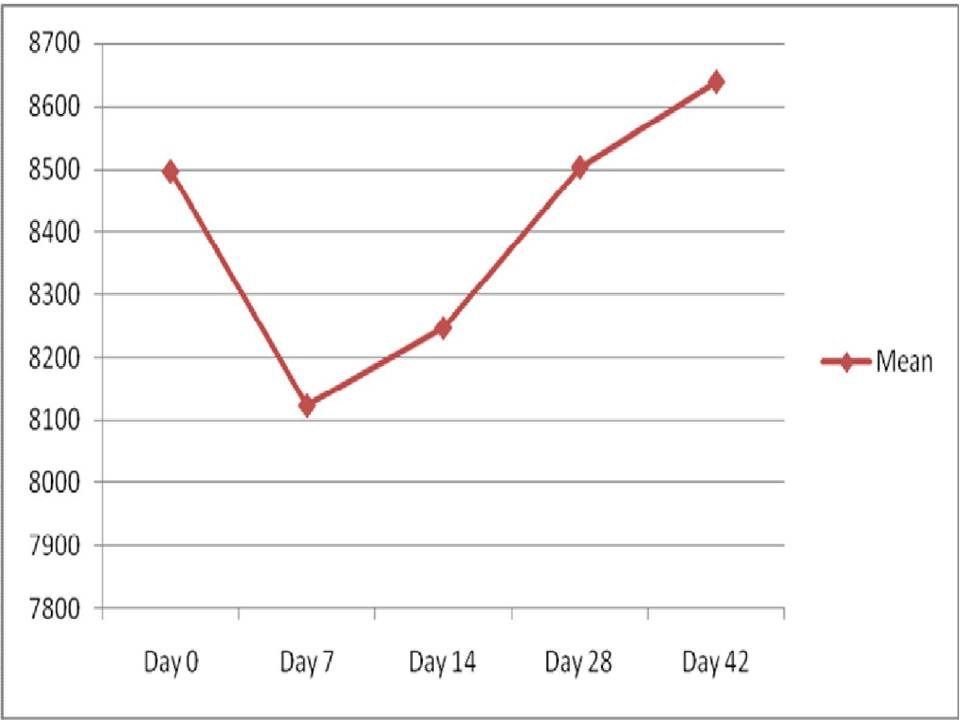
The comparison of mean total leukocyte count between the study group before the commencement of radiotherapy and the control group showed statistically an insignificant difference (p = 0.79). Comparison of total leukocyte count from day 0 to day 42 in the study group showed a significant decrease from day 0 to day 7 followed by an increase towards the end of radiotherapy [Table/Fig-7].
Comparison of total leucocyte count from day 0 to day 42
Discussion
Oral mucositis is a serious complication of cytotoxic chemotherapy and radiotherapy associated with significant morbidity, pain, odynodysphagia, dysgeusia, with subsequent dehydration and malnutrition reducing the quality of life of patients [19]. It is an integral part of radiotherapy and about 80% of the patients will develop pseudomembranous mucositis during radiotherapy [7,20]. Severe mucositis may necessitate an interruption of the course of radiotherapy [21] which must be prevented, because they may result in prolongation of treatment time and thus a reduction in therapeutic effect.
In the present study, patients were clinically evaluated for mucositis and scoring was done based on the WHO scale [18]. A significant increase in the severity of mucositis was seen during the course of treatment followed by a gradual decrease towards the end of radiotherapy. Trotti et al., studied more than 6,000 patients with head and neck squamous cell carcinoma who received radiotherapy with or without chemotherapy. The overall incidence of mucositis in this study was 80%, with 39% of cases being grade 3 or 4, which limited alimentation [10].
Galitis et al., noted that the prevalence of severe mucositis, pain and xerostomia during radiotherapy was 57%, 43% and 29% respectively and was significantly reduced to 33%, to 24% and to 18% at the end of radiotherapy [22].
The acute phase response is a cardinal response of the body to infection and trauma and may be the result of numerous immunologic reactions and inflammatory processes. It is indicated by changes in the plasma concentrations of a number of proteins synthesized by the liver, CRP being one of them. These changes in the plasma profile are fundamental to changes in ESR [23].
The present study showed a statistically highly significant difference (p<0.001) when a comparison of mean CRP between study group before the commencement of radiotherapy and the control group were made. The levels of CRP from day 0 to day 42 in the study group showed an increase towards the end of radiotherapy [Table/Fig-5]. This result is similar to a study done by Mohammed et al., on patients diagnosed with head and neck cancer receiving radiation treatment (RT) or radiation and concurrent chemotherapy (CRT). They concluded that there was a 4-fold increase in mean CRP with treatment and maximal levels of CRP occurred at the end of treatment (7 weeks) [24].
Such trends in serum values were also recorded by Cengiz et al., in patients with carcinoma of endometrium and cervix who were treated with surgery and postoperative radiotherapy. They inferred that acute phase response was present during the post radiotherapy phase with a significant rise in CRP at the end of radiotherapy and thus radiotherapy should be considered as a cause of increase in CRP level especially in clinical conditions where acute phase response is important [23].
Chatterjee et al., studied the effect of radiotherapy on tissue damage, innate and humoral immunity in malignant conditions by estimating complement C3, IgG and CRP. They concluded that the concentration of CRP was greater in cancer patients as compared to healthy ones. The study also revealed even more increase in CRP in every patient after radiotherapy [25].
There are late effects of radiation therapy which includes an ongoing inflammation in the body. It is evident that the mucosa bears only a small clinical spectrum of the side-effect conveyed by chemoradiation. Chemoradiation may incite an inflammatory process that is ongoing and the tissues may never return to normal completely. This leads to a persistence of adverse effects in many patients [26]. Cytokines play an important role in the inflammatory response. Systemic acute phase reactions are one of the most important actions in inflammation mediated by cytokines.
CRP is predominantly synthesized by the liver and is regulated by pro-inflammatory cytokines, primarily TNF-α and IL-6 [27]. There is a strong positive association between the duration and intensity of the stimulus (e.g. tissue injury) and the number of hepatocytes producing CRP. This is due to the activation of the hepatocytes in the direction of the blood flow which results in a higher peak level and also a protracted increase in serum CRP whenever the degree of the stimulus is stronger and longer [28]. Thus, the increase in CRP levels can be explained based on the ongoing inflammation mediated by cytokines which continuously activate the acute phase response in the body which is seen as a rise in CRP, a positive acute phase protein. Ki Y et al., evaluated the relationship between CRP levels and the grade of acute radiation induced mucositis in patients with head and neck cancer and concluded that a change of the mean CRP level was correlated with progression of mean grade of mucositis [29]. In our study, there was a correlation between CRP and the grading of mucositis during the initial weeks of the treatment protocol but towards the end, the CRP levels continued to show a rise [Table/Fig-5] inspite of the reduction in the severity of mucositis as recorded by the grading system.
In the present study, comparison of mean ESR between study group before the commencement of radiotherapy and the control group showed statistically a very highly significant difference (p<0.001). The ESR levels from day 0 to day 42 in the study group showed a significant increase till day 14 followed by a decrease towards the end of the treatment protocol [Table/Fig-6]. This was in correlation with the grading of mucositis which also showed an initial rise in the severity of mucositis followed by a decrease towards the end of treatment.
These values did not correlate with studies performed by other authors. Ki Y et al., in their study stated that ESR did not show any statistically significant relationship with the grade of acute mucositis [29]. According to Mohammed et al., there was over 2-fold increase in mean ESR levels with treatment. Maximal levels of ESR occurred at the end of treatment (7 weeks) [24]. Cengiz et al., stated that there was a significant rise in ESR at the end of radiotherapy. The sensitivity of ESR as a definite indicator in various disease processes can vary. The factors that can increase ESR are old age, higher levels in females, pregnancy, anaemia, elevated fibrinogen level and various technical errors. The factors that can decrease ESR are extreme leucocytosis, protein abnormalities, inadequate mixing of the sample, clot formation and various other technical errors [23].
In the present study, comparison of mean total leukocyte count between study group before the commencement of radiotherapy and the control group did not show a statistically significant difference (p = 0.79). Comparison of total leukocyte count from day 0 to day 42 in the study group showed a significant decrease from day 0 to day 7 followed by an increase towards the end of radiotherapy [Table/Fig-7]. As per the present study, there was no correlation between the total leukocyte count and the severity of mucositis.
The variation in total leukocyte count during the study was not in correlation with the study performed by Yang et al., [30]. They analysed weekly complete blood counts in patients receiving standard fractionated partial body radiation therapy for breast, prostate, lung, gynecological, or head and neck malignancies. Their conclusion was that the leukocytes decreased most dramatically during the first week of treatment (16% from pre-treatment to week 1 levels) and then at a rate of 3.3% per week from week 1 to week 7 [30].
Limitations of The Study
The absence of leucopenia recorded in the present study could probably be due to the inclusion of only head & neck radiotherapy patients and thus the absence of major bone marrow suppression. Thus, the effect of radiotherapy on leucocyte levels may not be accurately studied in such patients.
Conclusion
This study analysed the acute phase response in radiation induced mucositis in head and neck cancer patients undergoing chemoradiotherapy. The increase in CRP levels can be explained based on the ongoing inflammation mediated by cytokines which can continuously activate the acute phase response in the body. Both widespread and late effects do occur and the tissues may never return to normal completely.
The aggressive treatment of head and neck cancer requires an appreciation of the balance between toxicity and tumor control. The routinely used scoring systems to evaluate a combination of symptoms and direct mucosal observation, although accurate scoring may be clouded by improved symptom control with supportive care measures and improved pain management. The clinical manifestations of post chemo-radiation mucositis subsides after an expected healing phase but the biochemical profile can take a longer time to return back to normal.
Thus, inflammatory serum markers like CRP can be used as an objective measure to study the complexities of radiation mucositis. This warrants further exploration of the pathophysiologic process from a biochemical and immunobiologic perspective. This study looked into the levels of CRP as a major APP but evaluation of the rest of the spectrum of APPs and their interplay during the healing phase will be interesting and shed light into this clinical entity which affects the quality of life in patients with head and neck cancer.