Chlorhexidine gluconate is to date a gold standard, most thoroughly studied and most effective anti-plaque and anti-gingivitis chemical plaque control agent when addressing oral hygiene [5]. Nevertheless, this mouthwash has been reported to have a number of local side effects on its long term use like brown discoloration of teeth, some restorative materials and dorsum of tongue; taste perturbation; oral mucosal ulcerations and paraesthesia; unilateral/bilateral parotid swelling and enhanced supra-gingival calculus formation [6].
Essential oils are ideal for use in oral care products because they are both antibacterial and nontoxic–a rare combination. Mouth washes containing essential oils are used for many years in the prevention and treatment of periodontal disease. Recent studies have demonstrated that essential oil mouth washes are as effective as chlorhexidine mouthwash in inhibiting the plaque regrowth [7] by infiltrating the plaque biofilm, disrupting the cell wall of the pathogenic micro-organisms and ultimately killing them and constrain their enzymatic activity [8]. Essential oil mouth wash prevent bacterial aggregation, slows their multiplication and extract the bacterial endotoxins [9]. The mechanisms by which essential oils can inhibit microorganisms may be due to their hydrophobicity, due to which they get partitioned into the lipid bilayer of the cell membrane, rendering it more permeable, leading to leakage of vital cell contents [10]. Impairment of bacterial enzyme systems may also be a potential mechanism of action [11].
Therefore with a background of plaque control as a key factor for prevention of periodontal diseases, limited effectiveness of mechanical plaque control, long term side effects of chlorhexidine gluconate and linking the faith of people for herbal/natural products and potency of lemongrass oil the present study was conducted with an aim to compare the anti-plaque and anti-gingivitis efficacy of 0.25% lemongrass oil mouthwash with that of 0.2% chlorhexidine mouthwash.
Materials and Methods
In March 2013 this double blinded randomised controlled parallel designed clinical trial was planned with patients attending Kothiwal Dental College & Research Centre, Moradabad aged 25-45 years having gingivitis; following CONSORT guidelines for clinical trials. Based on a pilot study, to get a clinically significant difference between the groups the necessary sample size was estimated using sample size and power calculations developed by William D. Dupont and licensed under a Creative Commons Attribution-Non Commercial-No Derivs 3.0 United States License [15]. With 5% type I error and 20% type II sample size was estimated to be 15 subjects in each group, but originally 20 subjects were recruited in each group forecasting some amount of loss to follow-up during the course of the study.
Before starting, ethical clearance for the present study was obtained from the institutional ethical committee of Kothiwal Dental College & Research Centre, Moradabad, India and the examiner was calibrated so as to achieve a minimum kappa value of 0.80 for inter and intra-examiner consistency. In order to ensure this, 5 patients were randomly selected and examined & re-examined for the plaque index (PI) score and gingival index (GI) score. The kappa value for intra-examiner reliability was found to be 0.88 and 0.90 respectively. The examiner was trained to match the ability of a gold standard examiner, to check for this inter-examiner reliability testing was done for plaque index (PI) score and gingival index (GI) score of the same patients. The kappa statistics for this was found to be 0.85 and 0.87 respectively.
The inclusion criteria for the study were set as: patients with mild to moderate gingivitis. Patients with any systemic diseases, known allergic to lemongrass derivatives, received antibiotic treatment 6 months prior to study, inability to comply with the follow-up visit requirements, undergoing orthodontic treatment or any other treatment that may affect periodontal health, pregnant and nursing patients and patients who are suffering from oral disease that need emergency treatment like endo-perio lesion, periodontal abscess etc. were excluded.
So, a study sample of 60 patients attending Kothiwal Dental College & Research Centre, Moradabad with mild to moderate gingivitis and aged 25-45 years who fulfilled the inclusion & exclusion criteria and signed informed consent after being described about the nature, potential risks and benefits of their participation in the study were recruited.
All the 60 patients underwent oral prophylaxis and randomly divided into 3 equal groups by a periodontal expert who was not concerned with the study to ensure blinding by lottery method. They were also not aware of other parallel groups or mouthwashes used in the present study. After that the subjects were advised to follow the regime of their respective groups and were asked to report to the department again on the 14th and 21st day [Table/Fig-1].
Schematic representation of study design
0.25% Lemongrass oil mouthwash (n=20) :- Lemongrass oil mouthwash was prepared using the standard protocol in Department of Pharmacology, Kothiwal dental college & research centre. Patients were advised for regular use of 0.25 % lemongrass oil mouthwash (twice daily) for 1 minute and brushing (twice daily) for 2-3 minutes for 21 days.
0.2% Chlorhexidine mouthwash Group- (n=20):- Patients were advised for regular use of commercially available 0.2% chlorhexidine mouthwash (twice daily) for 1 minute and brushing (twice daily) for 2-3 minutes for 21 days.
Oral prophylaxis only Group (n=20):- Patients were advised for brushing (twice daily) for 2-3 minutes for 21 days.
Modified bass technique was demonstrated to the subjects and they were also supplied with new toothbrushes of same make and brand to maintain the uniformity at commence of the experimental period. The participants were instructed by the examiner on the use of mouthwash and brushing and regular performance was reinforced every 3 days through telephone calls to ensure compliance.
The oral examinations were conducted in the Department of Public Health Dentistry, Kothiwal Dental College and Research Centre, Moradabad. Gingival status & plaque accumulation at baseline was assessed followed by oral prophylaxis at the same visit. Subjects were again assessed after 14 & 21 days of intervention for plaque and gingival score as the time required for the development of gingivitis ranges from 10 to 21 days [16]. Gingival status was assessed by Gingival Index, Loe & Silness (1963), Dental plaque by Plaque Index, Silness & Loe (1964) [17,18].
Statistical Analysis
Statistical analysis was carried out using SPSS ver. 20.0 (SPSS, Inc., Chicago, IL, USA). Levene’s test for homogeneity of variance (p<0.05) was performed, as we assumed equity of variance was more important than an assumption of normality. A comparison of the mean differences of the GI and PI was analysed by a paired t-test and between the groups by one-way analysis of variance (ANOVA). Post-hoc test (Tukey’s Test) was done to identify the significant pairs. Level of significance was set at p ≤ 0.05 (95% confidence interval).
Results
All the 60 subjects completed the study. [Table/Fig-2] depicts the mean ± SD score for PI and GI of all the three groups at baseline, 14th and 21st day.
Mean PI & GI of all the groups at baseline, 14th day & 21st day
| Evaluation Period | Baseline | 14th Day | 21st Day |
|---|
| Intervention Groups | PI ± SD | GI ± SD | PI ± SD | GI ± SD | PI ± SD | GI ± SD |
|---|
| 0.25% Lemongrass Oil Mouthwash | 1.85 ± 0.27 | 2.19 ± 0.19 | 1.17 ± 0.33 | 1.76 ± 0.35 | 1.20 ± 0.19 | 1.59 ± 0.37 |
| 0.2% Chlorhexdine Mouthwash | 1.87 ± 0.30 | 2.23 ± 0.31 | 1.18 ± 0.26 | 1.86 ± 0.45 | 1.21 ± 0.28 | 1.67 ± 0.46 |
| Oral prophylaxis Only | 1.90 ± 0.30 | 2.18 ± 0.32 | 1.42 ± 0.33 | 1.98 ± 0.30 | 1.43 ± 0.36 | 1.99 ± 0.42 |
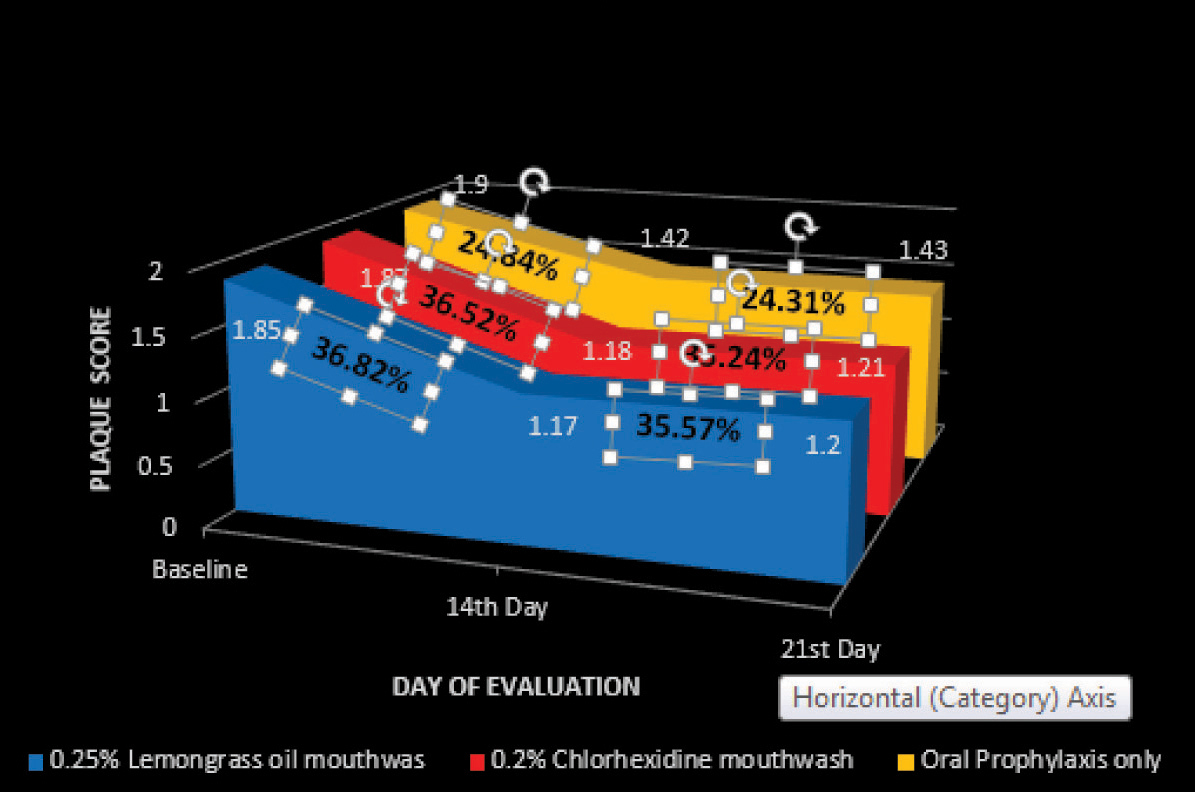
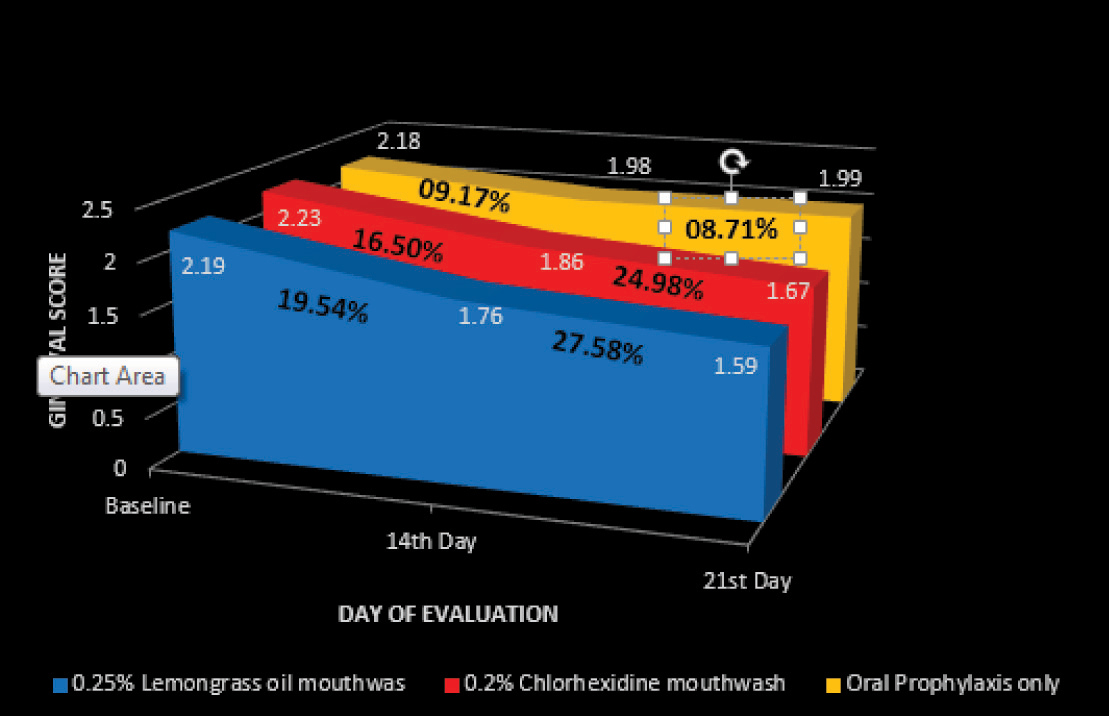
A greater reduction in mean PI and GI score was recorded in the 0.25% lemongrass oil mouthwash group at both 14th and 21st day followed by 0.2% chlorhexidine mouthwash group followed by oral prophylaxis only group. It was found that the mean reduction in PI and GI score after intervention in all the three groups to be statistically significant (p < 0.05) at both 14th and 21st day [Table/Fig-3,4 and 5].
Reduction in mean PI & GI from Baseline at 14th day & 21st day
| Intervention Groups | 0.25% Lemongrass Oil Mouthwash | 0.2% Chlorhexdine Mouthwash | Oral prophylaxis Only |
|---|
| PI | GI | PI | GI | PI | GI |
|---|
| Evaluation Period | Mean difference (%) | 95% CI | Mean difference (%) | 95% CI | Mean difference (%) | 95% CI | Mean difference (%) | 95% CI | Mean difference (%) | 95% CI | Mean difference (%) | 95% CI |
|---|
| 14th Day | 0.68* (36.82%) | 0.54 -0.83 | 0.43* (19.54%) | 0.30 -0.55 | 0.68* (36.52%) | 0.58 -0.79 | 0.37* (16.5%) | 0.24 –0.5 | 0.47* (24.84%) | 0.36 -0.58 | 0.20* (9.17%) | 0.13 -0.27 |
| 21st Day | 0.66* (35.57%) | 0.53- 0.79 | 0.60* (27.58%) | 0.47- 0.74 | 0.66* (35.24%) | 0.49- 0.83 | 0.56* (24.98%) | 0.419- 0.695 | 0.46* (24.31%) | 0.35- 0.58 | 0.19* (8.71%) | 0.13- 0.24 |
*p<0.05; Statistically Significant
Reduction in Plaque score on 14th and 21st day from base line
Reduction in Gingival score on 14th and 21st day from base line
On comparison between the groups, the mean PI score shows a statistically significant difference at both 14th (p=0.019) and 21st (p=0.017) day. It was found that for GI score this difference was not statistically significant at 14th day (p=0.192) but was statistically significant at 21st day (p=0.004) [Table/Fig-6].
ANOVA to compare between and within the groups at baseline, 14th and 21st day
| Evaluation Period | | Sum of Squares | Degree of Freedom | Mean Square | F | Significance |
|---|
| PlaqueScore | Baseline | Between GroupsWithin GroupsTotal | 0.184.8134.832 | 25759 | 0.0090.084 | 0.107 | 0.898 |
| 14th Day | Between GroupsWithin GroupsTotal | 0.8135.4776.290 | 25759 | 0.4060.096 | 4.230 | 0.019* |
| 21st Day | Between GroupsWithin GroupsTotal | 0.7194.6415.360 | 25759 | 0.3590.081 | 4.413 | 0.017* |
| GingivalScore | Baseline | Between GroupsWithin GroupsTotal | 0.284.4614.489 | 25759 | 0.0140.078 | 0.179 | 0.837 |
| 14th Day | Between GroupsWithin GroupsTotal | 0.4767.9898.465 | 25759 | 0.2380.140 | 1.699 | 0.192 |
| 21st Day | Between GroupsWithin GroupsTotal | 1.8088.44310.521 | 25759 | 0.9040.148 | 6.105 | 0.004* |
*p<0.05; Statistically Significant
[Table/Fig-7] depicts the results of the Tukey’s post-hoc test to get the significant pairs. A statistically significant difference was seen for PI on comparing among the groups; with significant pairs being 0.25% lemongrass oil mouthwash group versus oral prophylaxis only group and 0.2% chlorhexidine mouthwash group versus oral prophylaxis only group at both 14th and 21st day. For GI score this difference was statistically significant only at 21st day and the significant pairs being 0.25% lemongrass oil mouthwash group versus oral prophylaxis only group and 0.2% chlorhexidine mouthwash group versus oral prophylaxis only group.
Tukey (post-hoc) Test to get significant pairs
| Evaluation Period | 14th Day | 21st Day |
|---|
| Intervention Groups | Mean Difference (SE) | 95% CI | Mean Difference (SE) | 95% CI |
|---|
| PlaqueScore | Oralprophylaxis Only V/S 0.25% Lemongrass Oil Mouthwash | 0.25* (0.09) | 0.02 – 0.49 | 0.24* (0.09) | 0.02 – 0.45 |
| Oralprophylaxis Only V/S 0.2% Chlorhexdine Mouthwash | 0.24* (0.09) | 0.00 – 0.48 | 0.23* (0.09) | 0.01 – 0.44 |
| 0.2% Chlorhexdine Mouthwash V/S 0.25% Lemongrass Oil Mouthwash | 0.01 (0.09) | -0.22 – 0.25 | 0.01 (0.09) | -0.21 – 0.23 |
| GingivalScore | Oralprophylaxis Only V/S 0.25% Lemongrass Oil Mouthwash | 0.22 (0.12) | -0.07 – 0.50 | 0.40* (0.12) | 0.11 – 0.70 |
| Oralprophylaxis Only V/S 0.2% Chlorhexdine Mouthwash | 0.12 (0.12) | -0.17 – 0.40 | 0.32* (0.12) | 0.02 – 0.61 |
| 0.2% Chlorhexdine Mouthwash V/S 0.25% Lemongrass Oil Mouthwash | 0.10 (0.12) | -0.18 – 0.38 | 0.08 (0.12) | -0.20 – 0.38 |
*p<0.05; Statistically Significant.
Discussion
This prospective double-blinded parallel designed clinical trial was conducted to check the efficacy of lemongrass oil as a mouthwash and comparisons were made with gold standard 0.2% chlorhexidine mouthwash and oral prophylaxis only as control groups. To overcome the Hawthorne effect these control groups were chosen [19].
The results of the present study showed that there is a statistically significant reduction in PI score at both 14th and 21st day in all the three groups. The anti-biofilm activity of lemongrass oil can be attributed to the presence of various constituents such as citral, limonene, citronellal, β-myrcene, linalool and geraniol [20]. These tarpenes present in lemongrass oil alter cell permeability by penetrating between the fatty acyl chains making up the membrane lipid bilayers, disrupting lipid packing and changing membrane fluidity. These phenomena led to major surface alterations and morphological modifications, also reducing the adherence capacity of oral pathogens [21]. Since adherence represents a major step in biofilm formation, therefore, these agents might be used to prevent biofilm-associated infection [22].
In the 0.2% Chlorhexidine mouthwash group this reduction is due to attack of chlorhexidine on bacterial cell membrane, causing leakage and/or precipitation of the cellular contents; specifically, by binding to salivary mucins, which reduces pellicle formation and inhibits plaque colonization. It also binds to bacteria and hinders their absorption onto the teeth [23]. The statistically significant reduction in the oral prophylaxis only group, is explained by the fact that oral prophylaxis removes the retentive plaque from the tooth surface and it becomes difficult for the new plaque to be deposited over the smooth surface of the tooth that easily as it was before in the presence of plaque [24].
Reduction in PI score is more in both the mouthwashes group as compared to that of the oral prophylaxis only group may be due to the fact that chemical plaque control as an adjunct to mechanical plaque control provides better outcome [4]. While comparing between both the mouthwashes groups the results show a slight better picture for the 0.25% lemongrass oil mouthwash as compared to 0.2% chlorhexidine mouthwash because of its additional property of oil pulling and the protective layer of oil on the smooth surface of the tooth which makes plaque deposition bit difficult. The viscosity of the oil probably inhibits bacterial adhesion and plaque co-aggregation. The other possible mechanism might be the saponification or the “soap-making” process that occurs as a result of the alkali hydrolysis of fat [25]. It is accordance with the results of study done by Kukkamalla MA et al., [3].
The GI score also showed statistically significant reduction in all the three groups at both 14th and 21st day. This can be explained by the association of dental plaque with gingivitis; so with elimination of the main etiologic factor there is subsequent reduction in GI score [4]. Highest reduction was recorded in the 0.25% lemongrass oil mouthwash group; which is because of the additive anti-oxidant and anti- inflammatory effect of lemongrass oil [26]. In gingivitis the inflammatory infiltration mainly consists of lymphocytes, plasma cells and neutrophils, which affects the oxidative stress and anti-oxidant pattern of these tissues [27]. To overcome this oxidative stress and maintain homeostasis these tissues depends on natural anti-oxidants [28]; which is very well provided by the lemongrass oil [25].
Results of the present study cannot be directly compared with that of any other study because to the best of our knowledge, it is one of the first studies of its kind, evaluating the effectiveness of 0.25% lemongrass essential oil as a mouthwash on dental plaque and gingivitis. But lemongrass oil in other form had a positive effect in the therapeutic course of gingivitis [29]; and the findings in the present study are indirectly similar to them. Microbial recolonization of periodontal pockets can be prevented by the effective anti-inflammatory and anti-microbial activity of lemongrass oil which augmented the clinical resolution of gingival inflammation. Also, periodontal tissue destruction is prevented and healing is enhanced by the antioxidant activity of the same [29].
One of the non-enzymatic antioxidants found in every cell of the body is Glutathione, also known as sulfhydryl glutathione (GSH) plays an important role in protection against oxidative stress. According to Susanto SA et al, gargling with 2% and 4% concentrations of lemongrass essential oil increased the salivary GSH levels in moderate gingivitis patients, with the same potency as hexitidine 0.1%, So it can speed-up gingivitis healing process [30]. Antioxidants like that of lemongrass essential oil overcome the ill-effects caused due to Reactive Oxygen Species (ROS) activity. In inflammatory process like gingivitis GSH not only acts as an anti-oxidant but also an immune function modulator. It directly acts as free radical scavenger in detoxification of reactive oxygen and nitrogen species and also avoids the production of pro-inflammatory cytokines [31–33].
Anand et al., estimated antioxidant property of lemongrass oil by evaluating salivary and gingival crevicular fluid GCF superoxide dismutase and thiol levels before and after its administration and eventually its effectiveness as an active ingredient of mouthwash. According to their results Superoxide dismutase and thiol levels increased from its baseline score; along with reduction in gingivitis for all concentrations (0.1%, 0.25%, and 0.5%) of Lemongrass oil mouthwash when used in adjunct to non-surgical periodontal therapy. From the quoted results and also from the results of the present study it can be implied that the lemongrass oil mouthwash may have an adjunctive effect on the treatment outcome, when it is used along with non-surgical periodontal therapy [26,34].
Lemongrass essential oil could inhibit the growth of several kinds of microorganisms at a concentration less than or equal to 2%. The antioxidant activity of lemongrass oil is because of its contents such as citral (neral and geranial) and citronellal [30]. In the present study, both antimicrobial and antioxidant activities were achieved by using 0.25% lemongrass essential oil in the mouthwash formulation.
Citral can subside oxidative stress through GSH’s antioxidant system induction because of its steroisomer; neral and geranial [35]. It can also act via terminating the chain reaction of lipid metabolism by donating hydrogen to free radical [36]. Flavonoid; a chemical component of lemongrass oil has many biological activities; viz., antioxidant, anti-inflammatory, antimicrobial, antimutagenic and anti tumour [37]. So its activity can evade oxidation reaction along with reducing hydroxyl radical, peroxyl radical and superoxide [38].
Citral is not only an active component of lemongrass oil but also helps in formation of Vitamin A and C; which are secondary antioxidants to scavenge free radicals and also prevents damage by stopping chain reaction [39].
Limitations
Our study is limited in its under-powered evaluations. Probably from a larger scale study a better and precise comparison can be achieved. This study was conducted for a very short span also, so long term study could reveal more prospects of this mouthwash.
Conclusion
Within the limitations of the present study, it can be concluded that 0.25% lemongrass oil mouthwash with antibacterial, anti-inflammatory and antioxidant properties seems to be an attractive alternating agent that can be used as an adjunct to mechanical nonsurgical periodontal therapy for plaque control and gingivitis. As it is one of the very few studies on the use of lemongrass oil as an active agent in mouthwash, so further studies on it with various other parameters are needed to know more of its beneficial and unfavourable (if any) effects.
*p<0.05; Statistically Significant
*p<0.05; Statistically Significant
*p<0.05; Statistically Significant.