Comprehensive Evaluation of Cardiac Hydatid Using 256 Slice Dual Source CT: One Stop Shop
Neeraj Jain1, Sonali Sethi2, Nishant Gupta3, Vandana Goel4, Sunil Kumar Puri5
1 Senior Resident, Department of Radiology, G B Pant Hospital, New Delhi, India.
2 Senior Resident, Department of Radiology, G B Pant Hospital, New Delhi, India.
3 Senior Resident, Department of Radiology, G B Pant Hospital, New Delhi, India.
4 Assistant Professor, Department of Radiology, G B Pant Hospital, New Delhi, India.
5 Head and Director Professor, Department of Radiology, G B Pant Hospital, New Delhi, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Neeraj Jain, Room No-305, New Resident Doctors Hostel, GB Pant Hospital, New Delhi-110002, India.
E-mail: jain_neeraj2@yahoo.co.in
Hydatid disease results from infection with larval stage of Echinococcus granulosus tapeworm. Dogs and other canines are the definitive hosts; Human beings are common accidental intermediate hosts. Liver is the most common organ to be involved in this condition. Cardiac hydatid, seen in only 0.5 to 2% cases, is a rare entity because of myocardial contractility. Larvae reach the myocardium through coronary circulation. Among various locations of cardiac hydatid, due to its rich coronary arterial supply Left ventricle (LV) myocardium is the most common site of involvement followed by interventricular septum and right ventricle. Rare locations include pericardium, right atrium and left atrium. A 50-year-old woman presented with dyspnoea for 11 months, chest X-ray showed a well defined, homogenous left paracardiac mass, which is not separable from left heart border. Transthoracic echocardiography revealed a complex multicystic mass lesion abutting antero-lateral wall of left ventricle. Contrast enhanced computed tomography showed a well-circumscribed multicystic mass lesion with honeycomb appearance arising from myocardium of anterolateral wall of left ventricle. Indirect haemagglutination test for hydatid disease was positive. At surgery the cyst was seen to arise from LV myocardium. It was incised and grape like contents were evacuated. The cavity was irrigated with scolicidal solution. Thereafter, the cyst was marsupialised. Histopathological examination revealed grape like cyst contents consistent with the diagnosis of hydatid cyst.
Cardiac hydatid, Multi-detector CT, Grape like cysts
Case Report
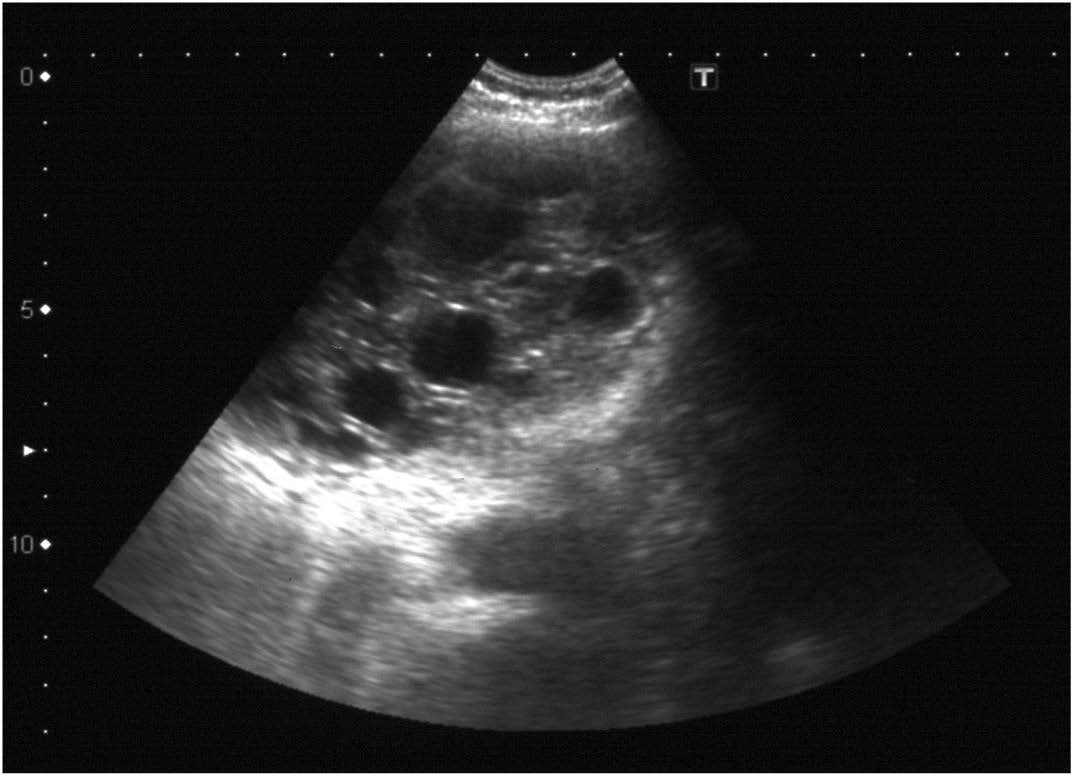
We report a case of isolated myocardial hydatid arising from anterolateral wall of left ventricle (LV) in a 50-year-old female, who presented with progressive exertional dyspnoea for 11 months. Physical examination was unremarkable. Chest radiograph revealed normal lungs and a well defined, homogenous left paracardiac mass. Its medial border was inseparable from left cardiac border. Echocardiography revealed a multicystic mass abutting the LV wall [Table/Fig-1]. There was mild mitral stenosis (MS) with moderate mitral regurgitation (MR). Ejection fraction was 69% with no regional wall motion abnormality. TLC was 8,500/mm3 with 1.2% eosinophils. Indirect haemagglutination test for hydatid disease was positive. History of contact with livestock or pet animals was negative.
Echocardiography showing a multicystic complex mass abutting the LV wall
On a 256-slice-dual source scanner (Somatom Definition Flash, Siemens, Germany), initially an ECG triggered coronary scan using prospective gating was done with 80ml 400mg % non-ionic iodinated contrast (Iomeron 400TM) at a rate of 5.5 ml/sec followed by saline bolus of 40 ml at same rate. This was immediately followed by another scan of entire chest for cyst morphology using standard chest parameters.
The radiation dosages delivered to the patient by the two scans were 1.274 mSv & 2.45 mSv respectively.
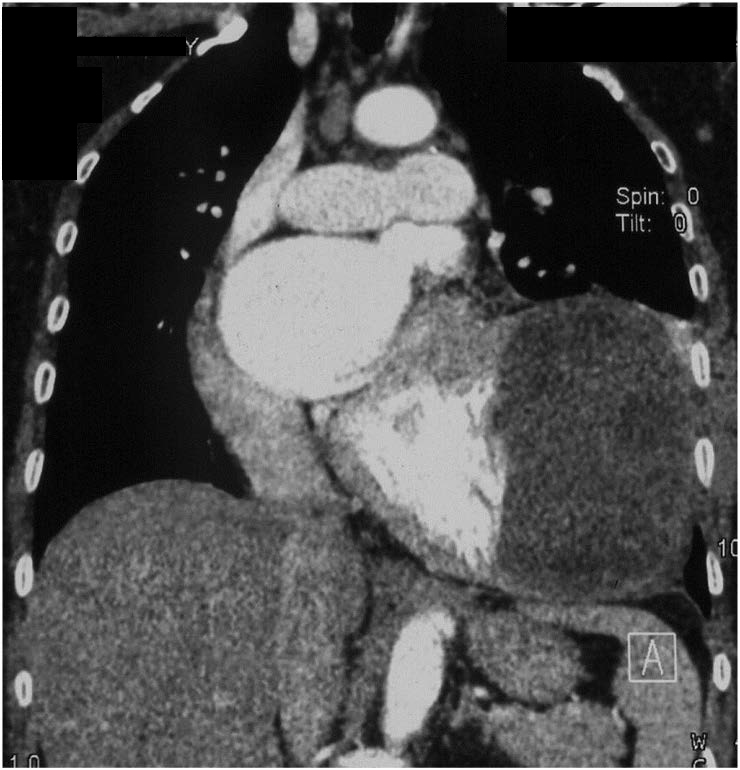
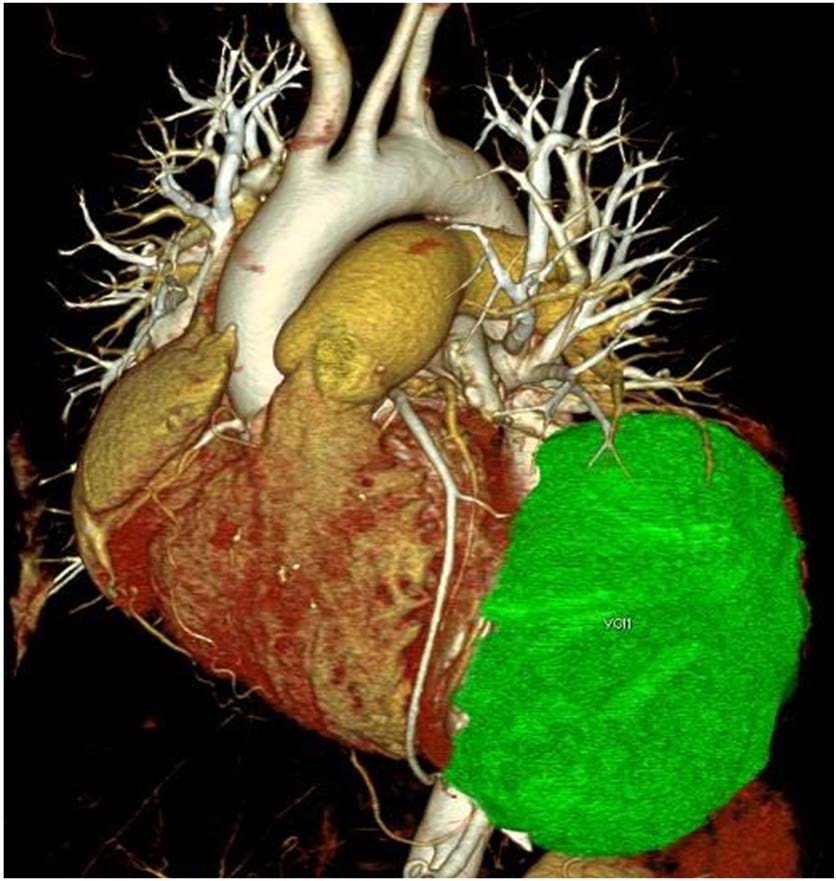
Data was post-processed to make MPR, MIP and VRT images of coronaries as well as standard images for chest. These revealed a 7.8cm x 6.9cm x 5.8cm size (volume 341 cc), oval, non-calcified, multicystic mass with honeycomb appearance arising from myocardium of anterolateral wall of LV [Table/Fig-2a]. Interventricular septum and posterior wall showed normally enhancing myocardium but the myocardium of anterolateral wall of LV was not discernable from the cyst wall [Table/Fig-2b]. All three coronary arteries were normal and the LAD showed [Table/Fig-3a,b] no displacement or extrinsic compression by the cyst. Pulmonary arteries and both lungs were normal [Table/Fig-3b]. Since all necessary information was available catheter coronary angiography was not performed.
CECT: Coronal MIP image showing a multicystic mass lesion with a ‘honeycomb appearance’ abutting the LV anterolateral wall
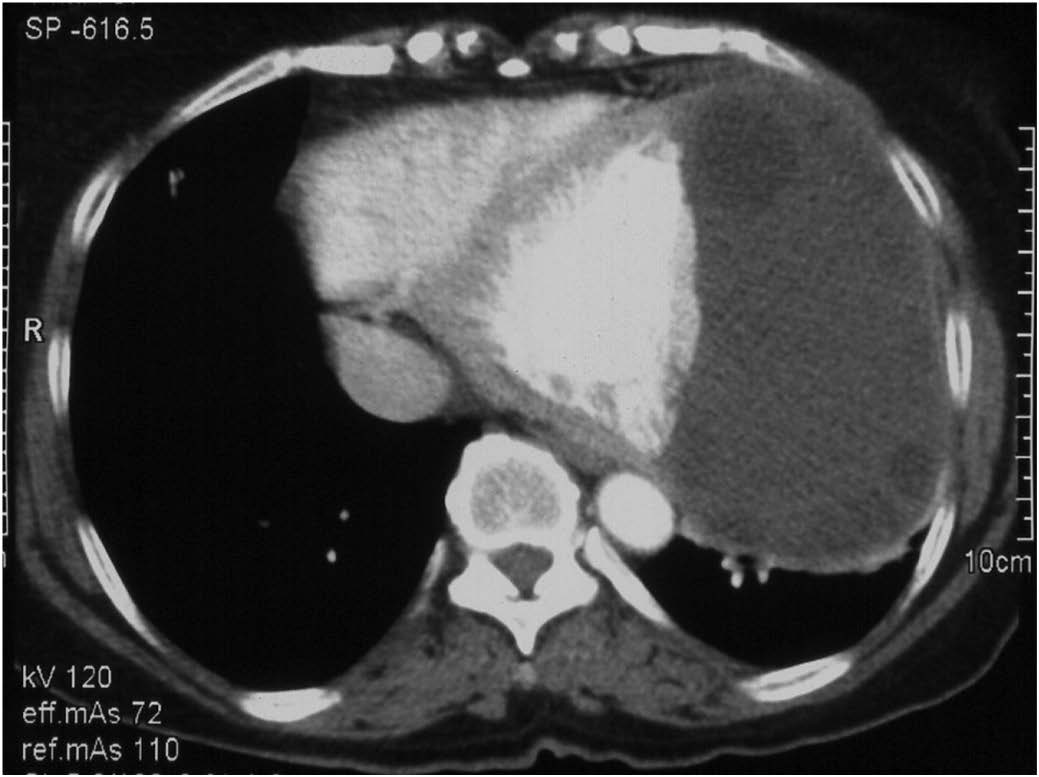
CECT Axial scan: The hydatid cyst wall is indiscernible from the anterolateral wall of LV. Note the well delineated interventricular septum and the posterior myocardial wall
CT Coronary angiography curved MIP image demonstrating LAD and the cyst
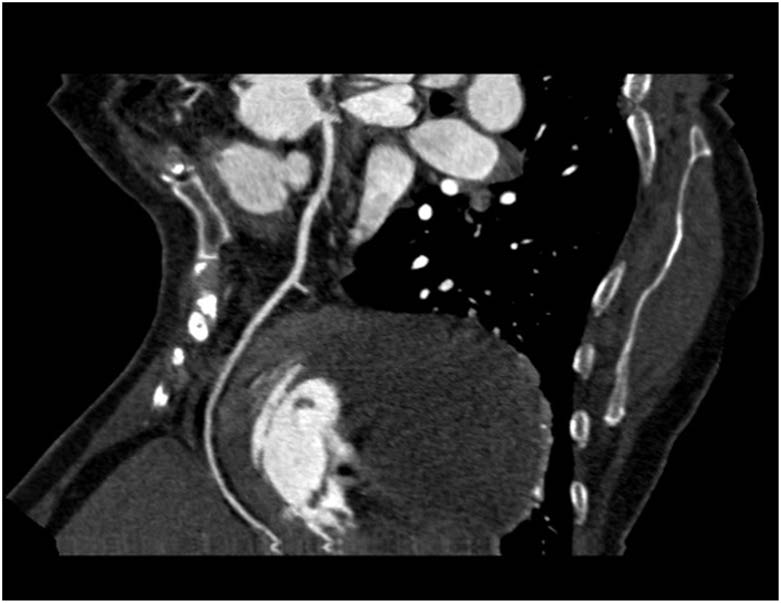
Colour coded VRT images beautifully demonstrates the cyst (green) in relation to the LAD (arrow). No compression or stenosis of the LAD noted. RCA is also visualized (arrowhead)
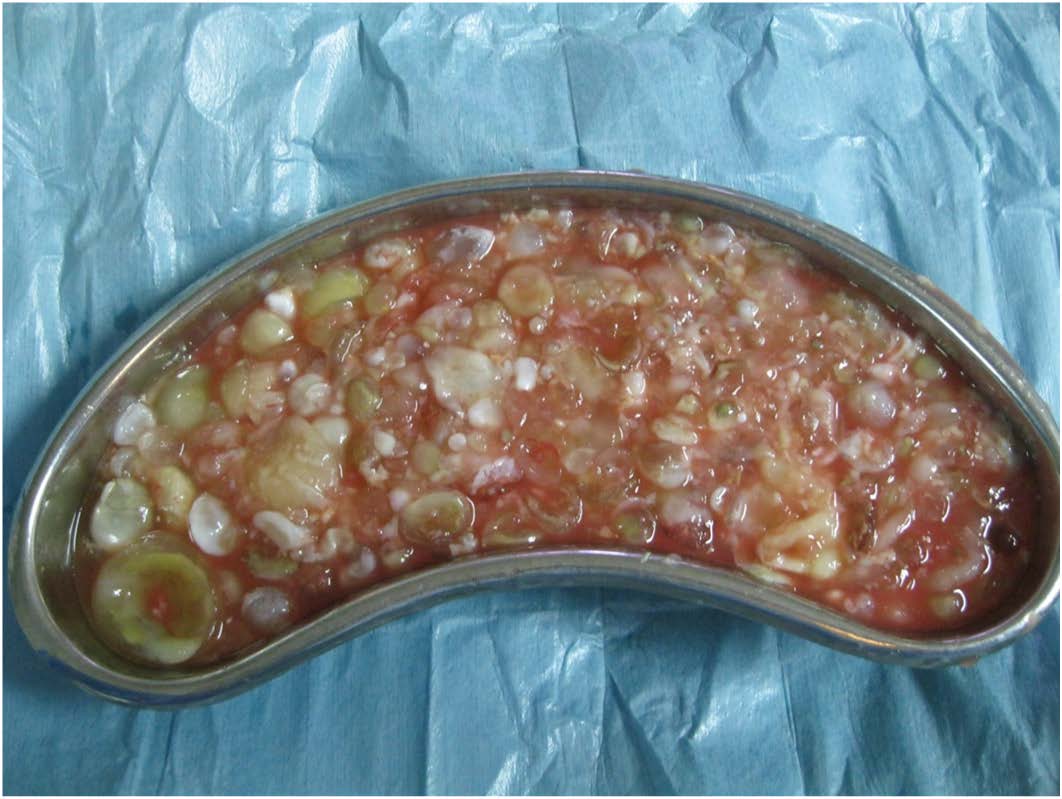
At surgery the cyst was seen to arise from LV myocardium. It was incised and grape like contents were evacuated [Table/Fig-4]. The cavity was irrigated with scolicidal betadine solution. Thereafter the cyst was marsupialised. The LV wall was adequately preserved and thus no ventricular wall reconstruction was done. Postoperative recovery was uneventful. Postoperative echocardiography showed disappearance of MR and MS suggestive of valvular abnormalities due to compressive effect by the cyst.
Discussion
Larval forms of the cestode Echinococcus granulosus cause hydatid disease, considered endemic in many countries including India [1]. Dogs and other canine are the definitive host, with sheep being the intermediate host. Human beings are common accidental intermediate hosts [2]. Common sites for hydatid cysts are liver (52-77%), lungs (9-44%), spleen (2-3%), kidney (1-2.5%) and brain (0.5-2%) [3]. Cardiac hydatid, seen in only 0.5 to 2% cases, is a rare entity because of myocardial contractility. Larvae reach the myocardium through coronary circulation. Among various locations of cardiac hydatid, LV myocardium is most common followed by interventricular septum and right ventricle. Rare locations include pericardium, right atrium and left atrium [4–7].
Cardiac hydatidosis usually presents with non-specific symptoms like chest pain, palpitations and dyspnoea in less than 10% patients [4–6,8]. However, it can lead to serious complications, such as ventricular arrhythmias, cardiac outflow obstruction, rupture, life threatening anaphylaxis, pulmonary embolism and cerebral embolism, cardiac tamponade and myocardial ischemia [9–11]. Serology for antibodies may be negative in 20% patients with hepatic cysts and nearly 40% with other cysts [8,12]. The definitive treatment of cardiac hydatid is surgical excision or marsupilization of cyst along with pre and postoperative albendazole therapy.
Different workers based on the sonographic appearances have classified hepatic hydatid cysts in many ways. WHO Informal Working Group on Echinococcosis (IWGE) classification divides the hepatic hydatid cysts into 5 types from CE1 to CE5 with CE3 divided into “A” and “B” categories. CE1 stage is completely anechoic cyst with double line sign, CE2 is multiseptated honey comb cyst, CE3A is cyst with detached membranes (water lily sign), CE3B is cyst with daughter cyst in solid matrix, CE4 is cyst with heterogeneous contents without daughter cyst and CE5 is solid components with calcified walls [13].
Differential diagnosis of cardiac hydatid on a chest radiograph remains wide with pericardial cysts, dilated atrium, left ventricular aneurysm and Morgagni hernia being the most frequent and important ones [14].
Echocardiography, CT and MRI are helpful modalities with respect to further characterization of these masses as they are able to demonstrate the internal consistency of these masses thereby differentiating between the solid and cystic components. Diagnosis of cardiac hydatid is straight forward in cases when the lesion shows multiloculated/multicystic appearance due to presence of daughter cysts as in type CE2 and CE3. Dilemma arises in cases of CE1/ anechoic cysts, which may mimic pericardial cysts in which case indirect haemagglutination tests, are corroborative to the diagnosis.
CT/MRI remains the primary diagnostic modality. However, newer extremely fast CT scanners with 64 or more detectors not only show exquisite details of cardiac hydatid but assessment of coronary and pulmonary arteries is also possible in one go. Therefore it can act as a single stage procedure, obviating the need for additional preoperative invasive catheter coronary angiography in case associated coronary artery disease is also suspected. Importance of relationship of coronary arteries with hydatid cyst has been well emphasized by D Sinan et al., who reported a similar case of myocardial hydatid cyst in a young patient [15]. In addition the overall radiation exposure to the patient by this technique is much lower compared to standard diagnostic CT scan and catheter coronary angiography. In our case total radiation dose delivered was significantly lower (2.5 times less) than what is expected had the patient undergone both a catheter coronary angiography and CECT chest. In this case we took a repeat chest scan with standard chest parameters as assessment of cyst morphology, cardiac wall enhancement as well as lung parenchyma would have been sub-optimal with dedicated protocol meant for coronary arteries assessment.
Thus, the advantages of reduced radiation dosage and complete assessment of the lesion along with assessment of coronaries in a single stage non-invasive examination should make a MDCT with capability of coronary angiography, modality of choice not only for cardiac hydatid but other cardiac masses also, where a preoperative invasive catheter angiography is otherwise required.
Conclusion
Myocardium is a rare site of occurrence for the hydatid cyst due to constant motion of the myocardium during the cardiac cycle. However, knowledge of this entity is a must amongst the radiologists and clinicians since it presents with non-specific symptoms. In order to achieve a greater confidence in diagnosing and adequate management of these cases, availability of a MDCT with 64 or more detectors can be a boon as it can not only confirm the diagnosis by providing exquisite details of the cyst but if required can also assess the coronary arteries in a single stage examination.
[1]. Mirrailes A, Bracamonte L, Pavie A, Bors V, Rabago G, Gandibakhch I, Cardiac echinococcosis. Surgical treatment and resultsJ Thorac Cardiovasc Surg 1994 107:184-90. [Google Scholar]
[2]. Akar AR, Eryılmaz S, Yazıcıolu L, Eren NT, Durdu S, Uysalel A, Surgery for cardiac hydatid disease: an Anatolian ExperienceAnadolu Kardiyol Derg 2003 3:238-44. [Google Scholar]
[3]. Dziri C, Haouet K, Fingerhut A, Treatment of hydatid cyst of the liver: where is the evidence?World J Surg 2004 28(8):731-36.Epub 2004 Aug 3 [Google Scholar]
[4]. Dighiero J, Canabal EJ, Aguirre CV, Hazan J, Horjales JO, Echinococcus disease of the heartCirculation 1958 17:127-32. [Google Scholar]
[5]. Tuncer E, Tas SG, Mataraci I, Tuncer A, Donmez AA, Aksut M, Surgical treatment of cardiac hydatid disease in 13 patientsTex Heart Inst J 2010 37:189-93. [Google Scholar]
[6]. Kabbani SS, Ramadan A, Kabbani L, Sandouk A, Nabhani F, Jamil H, Surgical experience with cardiac echinococcosisAsian Cardiovasc Thorac Ann 2007 15:422-26. [Google Scholar]
[7]. Moorthy N, Ananthakrishna R, Rajendran R, Gowda GSL, Bhat SPS, Nanjappa MC, Giant Cardiac Hydatid Cyst: An Uncommon Cause of CardiomegalyJournal of the American College of Cardiology 2013 62(16):e145 [Google Scholar]
[8]. Gossios KJ, Kontoyiannis DS, Dascalogiannaki M, Gourtsoyiannis NC, Uncommon locations of hydatid disease: CT appearancesEur Radiol 1997 7(8):1303-08. [Google Scholar]
[9]. Byard RW, An analysis of possible mechanisms of unexpected death occurring in hydatid disease (echinococcosis)J Forensic Sci 2009 54:919-22. [Google Scholar]
[10]. Nurkalem Z, Atmaca H, Kayacioglu I, Uslu N, Gorgulu S, Eren M, Hydatid disease involving the left ventricle: a case of unusual combinationInt J Cardiol 2006 112:e30-32. [Google Scholar]
[11]. Ben-Hamda K, Maatouk F, Ben-Farhat M, Betbout F, Gamra H, Addad F, Eighteen-year experience with echinococcosis of the heart: clinical and echocardiographic features in 14 patientsInt J Cardiol 2003 91:145-51. [Google Scholar]
[12]. Alehan D, Çeliker A, Aydıngöz U, Cardiac hydatid cyst in a child: diagnostic value of echocardiography and magnetic resonance imagingActa Paediatr Jpn 1995 37:645-47. [Google Scholar]
[13]. Turgut AT, Akhan O, Bhatt S, Dogra VS, Sonographic spectrum of hydatid diseaseUltrasound Q 2008 24:17-29. [Google Scholar]
[14]. Handler JB, Higgins CB, Warren SE, Computerized tomographic diagnosis of paracardiac massesWest J Med 1981 135:271-75. [Google Scholar]
[15]. Demirtas S, Yavuz C, Basyigit I, Firat U, Caliskan A, Myocardial hydatid cyst in a young male patient who feeds pet at home: a case reportCase Reports in Vascular Medicine 2012 2012doi: 10.1155/2012/413815 [Google Scholar]