Introduction
Alcohol use is a pervasive problem that is taking an increasing toll on the world’s population. The World Development Report [1] found that the alcohol related disorders affects 5-10% of the world’s population each year and accounted for 2% of the global burden of disease. Globally alcohol consumption has increased in recent decades, with most of the increase in developing countries. Increase is more in countries where use of alcohol is traditionally less on population level and methods of prevention, control or treatment are not easily available. ICMR bulletin estimated 62 million alcoholics in India which is as big as that of the population of France [2].
Heavy drinkers suddenly decreasing their alcohol consumption or abstaining completely may experience alcohol withdrawal (AW). Symptoms and signs of AW include mild to moderate tremors, irritability, anxiety, or agitation, among others. The most severe manifestations of withdrawal include delirium tremens, hallucinations, and seizures. These happen due to alcohol-induced imbalances in the brain which result in excessive neuronal activity if the alcohol is withheld [3].
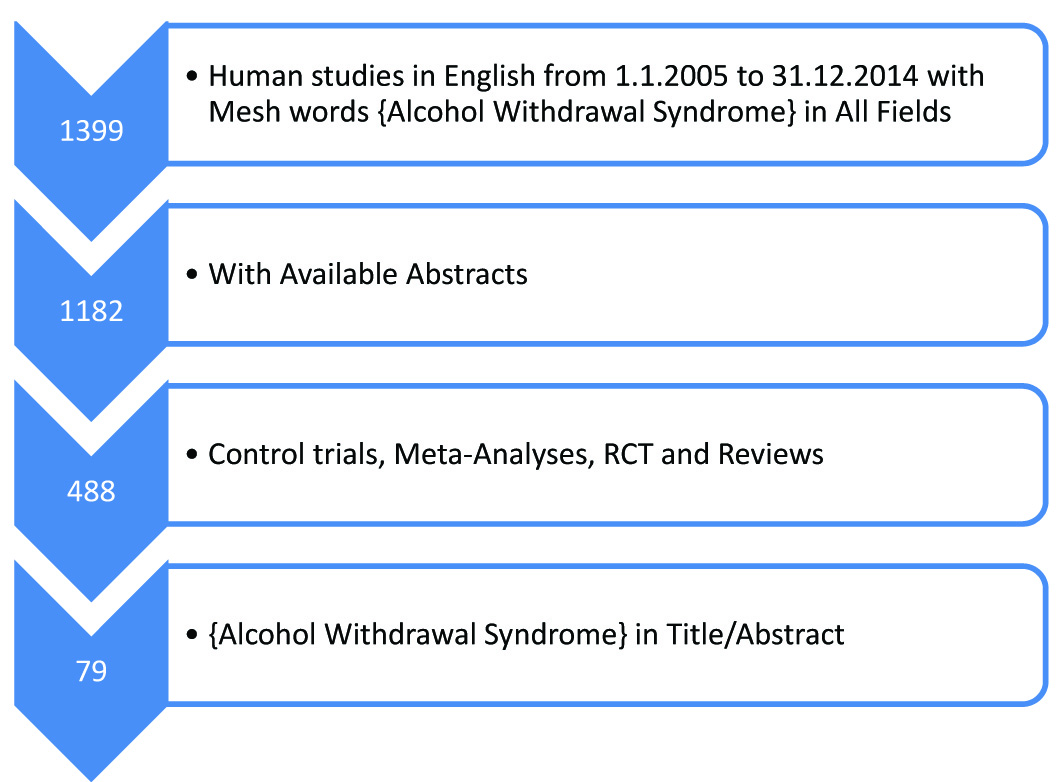
The aim of the present paper was to review the evidence base for the history, diagnosis and management of the alcohol withdrawal syndrome (AWS), with a focus on role of benzodiazepines in AWS. This review informs readers about pathophysiology of AWS, historical aspects, diagnosis and medications to be used for treating alcohol withdrawal, their dosing strategies to be used and different regimes of benzodiazepines. We searched Pubmed and MEDLINE database as detailed in the flowchart. After reading the abstract of these articles those relevant to clinical utility and management were shortlisted. The full text of the shortlisted articles were retrieved and read in full by the authors [Table/Fig-1]. Cross-references from selected studies were searched and further relevant articles were considered for inclusion. The data was synthesized and the relevant findings are discussed below.
Flowchart of article selection
Alcohol dependence syndrome: Concept and prevalence Alcohol abuse and dependence pose a major health problem worldwide with important social, interpersonal and legal implications. Dependence on alcohol is associated with both physiological symptoms such as tolerance and withdrawal, and behavioural symptoms such as impaired control over drinking [4]. It usually manifests when an alcohol dependent individual develops withdrawal symptoms after stopping alcohol, either due to family pressure, self-motivation, or difficulty in procuring alcohol.
Alcohol dependence is one of the most common psychiatric disorders, second only to major depression [5]. Data from the National Co-morbidity Survey and the NIMH Epidemiologic Catchment Program revealed that approximately 14% of the general population has a lifetime history of alcohol dependence. A recent National Household Survey of Drug Use in India [6] recorded alcohol use in only 21% of adult males. However, this figure cannot be expected to mirror accurately the wide variation that exists in a large and complex country such as India. The prevalence of current use of alcohol ranged from 7% in the state of Gujarat (officially under Prohibition) to 75% in Arunachal Pradesh.
The Alcohol Withdrawal Syndrome
The Alcohol withdrawal Syndrome (AWS) is one of the most common presentations of Alcohol Dependence Syndrome. AWS is a cluster of symptoms which occurs in alcohol-dependent people after cessation or reduction in heavy or prolonged alcohol use. The clinical presentation varies from mild to severe and the onset of symptoms typically occurs a few hours after the last alcohol intake. The most common manifestations are tremor, restlessness, insomnia, nightmares, paroxysmal sweats, tachycardia, fever, nausea, vomiting, seizures, hallucinations (auditory, visual, and tactile), increased agitation, and tremulousness. A minority of patients develop very severe alcohol withdrawal syndrome, including delirium tremens. These symptoms involve disturbances in a wide range of neurotransmitter circuits that are implicated in alcohol pathway and reflect a homeostatic readjustment of the central nervous system [7–9].
Pathophysiology
Historically, several mechanisms have been suggested to play a role in the development and etiology of AWS. Initially, the researchers thought that withdrawal might be caused by the nutritional deficiencies [10,11]. Some of the complications of withdrawal (e.g., seizures) were thought to result directly from alcohol use or intoxication [12]. Although alcohol dependent persons exhibit many metabolic and nutritional disturbances, overwhelming laboratory and clinical evidence presently indicates that the constellation of signs and symptoms known as AWS are caused by interruption of the constant exposure of the Central Nervous System (CNS) to alcohol itself.
Long-term alcohol consumption affects brain receptors, which undergo adaptive change in an attempt to maintain normal function. Some key changes involve decrease in both brain gamma-amino butyric acid (GABA) levels and GABA-receptor sensitivity [13,14] and activation of glutamate systems [15], which leads to nervous system hyperactivity in the absence of alcohol. Alcohol potentiates GABA’s inhibitory effects on efferent neurons, thereby suppressing neuronal activity. With chronic alcohol exposure, GABA receptors become less responsive and higher alcohol concentrations are required to achieve the same level of suppression, which is termed ‘tolerance’.
Alcohol also acts on N-methyl-D-aspartate (NMDA) receptor as an antagonist, thereby decreasing the CNS excitatory tone. Therefore, chronic use of alcohol leads to an up regulation of glutamate to maintain CNS homeostasis. Even when alcohol is no longer present in this adapted system, the GABA receptors remain less responsive; leading to an imbalance in favour of excitatory neurotransmission as the CNS excitation mediated by glutamate is left unopposed [3]. This CNS excitation is clinically observed as symptoms of alcohol withdrawal in the form of autonomic over activity such as tachycardia, tremors, sweating and neuropsychiatric complications such as delirium and seizures.
Dopamine is another neurotransmitter that is involved in alcohol withdrawal states. During alcohol use and the increase in the dopamine levels in CNS contribute to the autonomic hyper arousal and hallucinations. Withdrawal seizures are also thought to result from a lowering of seizure threshold due to kindling [16].
Diagnosis of Alcohol Withdrawal Syndrome
The alcohol withdrawal syndrome is diagnosed after a proper history and a thorough clinical examination. The diagnosis requires adequate history of the amount and frequency of alcohol intake, the temporal relation between cessation/reduction of alcohol intake and the onset of withdrawal symptoms. Withdrawal symptoms usually start around 6 hours of alcohol cessation. When the onset of withdrawal like symptoms or delirium is after 1 week of complete cessation of alcohol, the diagnosis of AWS becomes untenable, regardless of the amount and severity of alcohol dependence. For establishing a diagnosis of AWS, following conditions need to be fulfilled [17,18]:
A clear evidence of recent cessation or reduction of alcohol after previous high dose regular use.
Symptoms of alcohol withdrawal seen cannot be accounted for by any medical or another mental disorder.
Significant distress or decline in functioning in socio-occupational or other important areas due to the withdrawal symptoms.
The common AWS noted in patients presenting to clinics are anxiety, tremors of body and hands, elevated blood pressure, tachycardia, insomnia, elevated body temperature, sweating, hallucinations, dilated pupils nausea, disorientation, irritability, headache and grand mal seizure [17]. However, the signs and symptoms of AWS vary over time and may cause confusion. A time based presentation of AWS symptoms is described in [Table/Fig-2] [19,20]. The patient’s condition must be reviewed from time to time for the appearance of signs of medical or neurological illness which may not have been evident at admission but may develop subsequently.
Symptoms of Alcohol Withdrawal Syndrome
| Time of Appearance after Cessation of Alcohol Use | Symptoms |
|---|
| 6 to 12 hours | Minor withdrawal symptoms: insomnia, tremors, anxiety, gastrointestinal upset, headache, diaphoresis, palpitations, anorexia, nausea, tachycardia, hypertension |
| 12 to 24 hours | Alcoholic hallucinosis: visual, auditory, or tactile hallucinations |
| 24 to 48 hours | Withdrawal seizures: generalized tonic-clonic seizures |
| 48 to 72 hours | Alcohol withdrawal delirium (delirium tremens): hallucinations (predominately visual), disorientation, agitation, diaphoresis |
Objective assessment of severity of alcohol withdrawal may be done through scale based measurements. One of the reliable scales in common clinical practices is the Clinical Institutes Withdrawal Assessment - Alcohol Revised (CIWA-Ar) scale [21]. It is used to measure the severity of alcohol withdrawal in a patient diagnosed to have AWS. The CIWA-Ar is a 10-item scale used to quantify the severity of alcohol withdrawal. It can also be used to monitor withdrawal and medicate accordingly. The CIWA-Ar has high inter-rater reliability (r > 0.8) and constructs validity. Scoring is done for each item by the clinician using a Likert-type scale (0–7 in most cases) and maximum possible total score is 67. The evaluation is easy and takes less than two minutes. However, the scale is not a diagnostic tool [22]. It has also been found useful in Indian setting [23]. Scores of 0-8 indicate absent to minimal withdrawal, scores of 9-15 indicate moderate withdrawal and scores of 16 or more indicate severe withdrawal (impending DT) [24].
Pharmacotherapy for Alcohol Withdrawal
Over the years, the treatment for alcohol detoxification has evolved from the use of gradual weaning schedule of alcohol itself to the use of benzodiazepines and the newer miscellaneous drugs. Prompt pharmacological treatment is indicated in all cases of AWS, as non-treatment or under treatment can be fatal [25,26]. Benzodiazepines are safe, effective and the preferred treatment for AWS. The best-studied benzodiazepines for AW treatment are diazepam, chlordiazepoxide, and lorazepam [24,27].
Goals of Detoxification
Three goals of drug and alcohol detoxification as described by the American Society of Addiction Medicine (ASAM) are as follows [28]:
“To provide a safe withdrawal from the drug(s) of dependence and enable the patient to become drug-free”.
“To provide a withdrawal that is humane and thus protects the patient’s dignity”.
“To prepare the patient for on-going treatment of his or her dependence on alcohol or other drugs.”
Setting For Detoxification
Before the 1980’s, AWS was generally treated in an inpatient setting. Today, most detoxifications take place on an outpatient basis. A review by Abbott et al., in 1995 suggested that fewer than 20% of patients undergoing AWS detoxification required admission to an inpatient unit. Most importantly no reports of serious medical complications among AWS outpatients were found in this review except that one patient suffered a seizure after the start of detoxification [29]. However, Myrick and Anton (1998) suggested that the inpatient detoxification provided the safest setting for the treatment of AW, because it ensured that patients would be carefully monitored and appropriately supported. Compared with outpatient facilities, inpatient clinic may provide better continuity of care for patients who begin treatment while in the hospital. In addition, inpatient detoxification separates the patient from alcohol-related social and environmental stimuli that might increase the risk of relapse [30].
Despite the lack of research-based criteria, certain factors suggest that a patient should receive inpatient treatment. These factors include a history of significant alcohol withdrawal symptoms, high levels of recent drinking, a history of withdrawal seizures or DTs (Delirium Tremens), and the co-occurrence of a serious medical or psychiatric illness [31,32]. Predictors of severe alcohol withdrawal (Withdrawal Seizure or Delirium Tremens) should be taken into account and are listed in [Table/Fig-3] [33,34]. Out-patient treatment can be offered to patients who don’t have these risk factors and this decision relies on the withdrawal signs. Pharmacotherapy may not be needed in all cases of mild alcohol withdrawal syndrome. These patients can be managed by supportive care and observation for up to 36 hours, after which they are unlikely to develop withdrawal symptoms.
Predictors of severe alcohol withdrawal (withdrawal seizure or Delirium Tremens)
| 1. | Older age |
| 2. | Past history of DT or alcohol withdrawal seizure |
| 3. | Severe withdrawal symptoms at initial assessment |
| 4. | Co-morbid medical or surgical illness |
| 5. | Presence of dehydration |
| 6. | Electrolyte disturbances (hyponatremia or hypokalemia) |
| 7. | Deranged liver enzymes |
| 8. | The presence of structural brain lesions |
General Principles of Supportive Care
It is essential to provide comfort and relaxation for patients presenting for alcohol detoxification. They should preferably be kept in a room which is quiet and has minimal stimulation and low lighting. Dehydration is an important component of AWS and should be given emergency check up. There should be immediate intravenous access for all patients with seizures or DT. If dehydrated, intra venous fluids should be started. Adequate sedation should be provided to calm the patient as early as possible. Restraints should be avoided, however, may be used as required in order to prevent injuries due to agitation or violence. Electrolyte imbalances must be promptly corrected after investigations. Vitamin B1 (Thiamine) supplementation helps to prevent Wernicke’s encephalopathy (WE) and should be given orally or intramuscularly to all the patients. Adequate nutrition must be ensured with balanced nutrients.
Cost of Management
The choice of treatment setting for alcohol detoxification has important cost implications. Hayashida and colleagues (1989) found outpatient alcohol detoxification to be considerably less costly than inpatient treatment ($175 to $388 versus $3,319 to $3,665, respectively) [35]. To some extent, the higher cost of inpatient treatment reflects the occurrence of more severe symptoms of AW as well as more co-occurring medical problems among hospitalized patients compared to ambulatory patients.
Drugs Used for Detoxification
1. Historical: Detoxification with Alcohol
Alcohol was itself used as a detoxifying agent through ‘gradual weaning’ that commanded support in the 18th and early 19th centuries [36]. By the mid-19th century, the Temperance movement strongly influenced the way habitual drunkenness is conceptualized and had widened its focus to condemnation of all alcoholic beverages. This made it difficult to advocate ‘gradual weaning’ as a justifiable intervention. A literature search (Medline, Cochrane, EmBase, Psycinfo and DARE) found just two recent reports concerning the use of alcohol for alcohol detoxification [37,38], although there are reports in other medical specialties [39].
Richard Saitz suggested that Alcohol should not be used to treat withdrawal for several reasons [3]. First, using alcohol as a treatment would promote its acceptability to the alcoholic. Second, alcohol has known toxic effects (e.g., impairing the function of the liver, pancreas, and bone marrow) that are not shared by the safer benzodiazepines. Third, in one clinical study, alcohol was inferior to the benzodiazepine, chlordiazepoxide [38].
2. Benzodiazepines
Benzodiazepines (BZD) are the mainstay of treatment in alcohol withdrawal. Benzodiazepines are safe, effective and the preferred treatment for AWS. Benzodiazepines are cross-tolerant with alcohol and modulate anxiolysis by stimulating GABA-A receptors [24]. During withdrawal from one agent, the other may serve as a substitute. They are proven to reduce withdrawal severity and incidence of both seizures and delirium tremens (DT) [40–42].
The ideal drug for alcohol withdrawal should have a rapid onset and a long duration of action, a wide margin of safety, a metabolism not dependent on liver function, and absence of abuse potential [43]. Various BZDs offer many of these advantages. BZDs have been found effective in: 1) preventing agitation and alcohol withdrawal seizures; 2) preventing delirium tremens; and 3) as cross-tolerant agents with ethanol. BZDs, owing to their wide margin of safety and low potential to produce physical dependence and tolerance in short-course therapy, are therefore very, effective in the treatment of alcohol-withdrawal syndrome. They are the drugs of choice for alcohol withdrawal [44].
Holbrook et al., did a meta-analysis of benefit of BZD in the treatment of acute alcohol withdrawal through randomized controlled trials in MEDLINE and the Cochrane Controlled Trials Registry [45]. They found that BZD were superior to placebo (common odds ratio {OR} 3.28, 95% confidence interval {CI} 1.30–8.28). Data on comparisons between benzodiazepines and other drugs, including α-blockers, carbamazepine and clonidine could not be pooled, but none of them was found to be superior to benzodiazepines. Another meta-analysis concluded that BZD reduce withdrawal severity, reduce incidence of delirium and seizures [24]. Hence, Benzodiazepines are suitable agents for alcohol withdrawal. The choice among different agents should be guided by rapidity of onset, duration of action and also the cost. Clonidine, beta-blockers, carbamazepine, and neuroleptics are not recommended as monotherapy though may be used as adjunct.
• Choice of Benzodiazepine
All subclasses of benzodiazepines appear to be equally effective in treating AWS [24]. Therefore, choosing a benzodiazepine depends on selection of preferred pharmacokinetic properties in relation to the patient being treated. The most commonly used benzodiazepines for alcohol detoxification are chlordiazepoxide, diazepam (long acting) and lorazepam, oxazepam (short/intermediate acting).
Saitz et al., suggested that long acting agents like diazepam and chlordiazepoxide which have greater half-life (i.e., for up to several days) can provide a smooth course of treatment without the risk of rebound symptoms (e.g. Seizures) that occur late during withdrawal as blood levels are reasonably uniform across the course of the day [3]. However, a disadvantage of these two drugs is that the dependence on demethylation and hydroxylation metabolic pathways, the long half-lives, and the presence of active metabolites make it likely that drug accumulation will occur in patients with liver disease. The benzodiazepine equivalents for 5 mg diazepam are 25 mg chlordiazepoxide, 1 mg lorazepam and 15 mg oxazepam.
Short-acting (i.e., for several hours) benzodiazepines like lorazepam should be used in patients with severe liver dysfunction and in patients who are at high risk of experiencing serious medical consequences following sedation, such as people with severe lung disease or elderly patients as it has no active metabolites and its metabolism is not much affected in liver. Short-acting benzodiazepines probably are efficacious as well but are associated with a greater risk of rebound symptoms. To prevent recurrence of withdrawal symptoms, these agents must be given in gradually decreasing doses/tapering doses before they can be discontinued.
• Regimens for alcohol detoxification
Three regimens for alcohol detoxification using benzodiazepines are most commonly followed [26],
Fixed tapering dose regimen (FTDR) – fixed doses of benzodiazepines are administered at scheduled intervals regardless of symptom severity. The initial doses are decided based upon the presenting severity of withdrawal and the time since last intake. This is best suited for out-patient setting where close monitoring is not possible [24]. Consequently, a fixed-dose regimen may be preferred in admitted patients if CIWA-Ar scores cannot be accurately performed (i.e. lack of training, outpatient care setting, co-morbid medical or psychiatric illnesses or use of medications that may affect CIWA-Ar measurements) [24].
Symptom triggered regimen (STR) – benzodiazepines are administered according to the withdrawal symptoms as assessed by withdrawal rating scales e.g. CIWA- AR. The ratings are done at a fixed schedule and drug doses are administered as per withdrawal severity. It needs training in applying scales and trained personnel. A symptom-triggered regimen is preferred in most cases of AWS because it results in the administration of less total medication and shorter duration of treatment [46,47]. This regimen may also reduce the risk of under medicating or over medicating a patient since dosing is based upon withdrawal symptoms [48]. The efficacy of symptom-triggered regimens, however, depends on the validity of patient assessment.
Loading dose regimen (LDR) - These regimens use long acting benzodiazepines to reduce the risk of complications such as seizures and delirium. An oral loading dose of 20 mg diazepam given every 2 hours was found useful in treating alcohol withdrawal. The withdrawal severity and the clinical condition needs to be monitored before each dose [49,50].
Mayo-Smith and Saitz and O’Malley formulated a treatment regimen in accordance with CIWA–Ar score severity [24,51]. According to these authors, patients with mild withdrawal symptoms (i.e., CIWA–Ar scores of 8 or less) and no increased risk for seizures can be managed without specific pharmacotherapy. Successful non-pharmacological treatments include frequent reassurance and monitoring by treatment staff in a quiet, calm environment. Patients who experience more severe withdrawal (CIWA-Ar scores ≥ 8) should get pharmacotherapy to manage their symptoms and lower the risk of seizures and DT’s.
• Efficacy of fixed tapering dose regimens over symptom triggered regimens
A few International studies tried to compare the different regimens of benzodiazepines used for alcohol detoxification but there is a paucity of Indian literature comparing the fixed tapering dose and the symptom triggered regimens of benzodiazepines inspite of alcohol abuse being such a huge public health problem.
Reoux and Miller in their retrospective analysis using treatment charts of patients hospitalized for uncomplicated alcohol withdrawal found that patients detoxified using a CIWA-Ar based protocol received significantly fewer chlordiazepoxide milligram equivalents over shorter durations than patients managed by other detoxification methods [52].
The advantage of the STR lies in the fact that detoxification is monitored through a standardized scale that results in administration of less benzodiazepines for a significantly shorter duration thereby reducing the cost to the patient as well as to the hospital. Day et al., concluded that STR is acceptable to both patients and staff and is potentially a useful technique for busy acute psychiatric wards [53]. Cassidy et al., reported that symptom-triggered approach reduced cumulative benzodiazepine dose and length of stay in an emergency department set up [54]. Similarly, other studies have also shown that STR reduces the benzodiazepine doses and duration of detoxification. Studies have been conducted on oxazepam [47], chlordiazepoxide [46] and chlormethiazole [55].
Jaeger T et al., conducted a retrospective analysis to assess the effectiveness usual care for AWS vs. the symptom-triggered therapy in patients admitted to the general medical services. They concluded that STR is an effective treatment for AWS in medical inpatients. Although it did not result in shorter duration of treatment, the STR was associated with a decreased occurrence of delirium tremens, the most severe and life-threatening complication of AWS [25].
An Indian study comparing the STR and FTDR of lorazepam for alcohol detoxification in 63 indoor patients found that STR resulted in shorter duration of treatment and lower total doses of medication. This double blind randomized controlled trial found STR to be as safe as the fixed tapering dose [56].
The review suggests that benzodiazepines are the preferred drugs for alcohol detoxification and all the benzodiazepines have proved similar efficacy for detoxification.
3. Anticonvulsant drugs
BZD’s are the drugs of choice for AWS in most of the treatment settings; however, anti-convulsant drugs may represent suitable alternatives. There are several potential advantages to using anti-convulsant drugs. Use of an anti-convulsant drug decreases the probability of a patient experiencing a withdrawal seizure, thereby reducing the complications of AWS. Anti-convulsant drugs also reduce craving. Anti-convulsant drugs have been shown to block kindling in brain cells. Anti-convulsant drugs do not appear to have abuse potential. Anti-convulsant drugs have been effectively used to treat mood disorders, which share some symptoms with AWS, including depression, irritability, and anxiety. The propensity of anti-convulsant drugs to cause sedation is much less as compared to BZD’s [30].
Carbamazepine has been shown to be superior in ameliorating global psychological distress and reducing aggression and anxiety compared to oxazepam [57]. Carbamazepine was also reported to be an effective alternative to benzodiazepines in the treatment of alcohol withdrawal syndrome in patients with mild to moderate symptoms [58]. Carbamazepine also appeared to decrease the craving for alcohol after withdrawal. Carbamazepine was found superior to benzodiazepines in prevention of rebound withdrawal symptoms and reducing post-treatment alcohol consumption, especially in patients who had multiple repeated withdrawals [59]. Carbamazepine use, however, has been limited due to its interaction with multiple medications that undergo hepatic oxidative metabolism, making it less useful in older patients and patients with medical co-morbidities [60]. Also, carbamazepine has not been evaluated for treating delirium tremens.
Reoux et al., and Malcolm et al., concluded that Valproic acid significantly affects the course of alcohol withdrawal and reduces the need for treatment with a benzodiazepine [61,62]. These two double-blind, randomized studies showed that patients treated with Valproic acid for 4 to 7 days dropped out less frequently, had less severe withdrawal symptoms including fewer seizures, and required less oxazepam than patients receiving either carbamazepine or placebo. Although effective, Valproic acid use may be limited by side effects—somnolence, gastrointestinal disturbances, confusion, and tremor—which are similar to alcohol withdrawal symptoms, making assessment of improvement difficult.
Gabapentin, which has structural similarity to GABA, is shown to be effective in the treatment of alcohol withdrawal [63,64]. Its low toxicity makes it a promising agent. Gabapentin was as effective as lorazepam in a randomized, double blind controlled study on 46 in-patients with alcohol withdrawal in the treatment of acute mild to moderate AWS [65]. Vigabatrin, an anticonvulsant agent, which irreversibly blocks GABA transaminase, showed improvement in withdrawal symptoms after only three days of treatment and is a promising agent for detoxification [66].
4. Adrenergic medications
Adrenergic medicines (centrally acting alpha-2 agonists like clonidine; and antagonist like propranolol), which alter the function of adrenergic receptors, are thought to significantly improve symptoms of AWS, especially autonomic symptoms, by reducing elevated pulse and blood pressure [51]. There is no evidence that these medications prevent or treat delirium or seizures. Adrenergic medications are of value largely as adjuncts to BZD’s in the management of AWS. These medications also may be useful in outpatient settings, where the abuse liability of BZD’s by patients is difficult to monitor or prevent and where AWS symptoms are generally less severe than among inpatient populations [67].
5. Barbiturates
Barbiturates such as phenobarbitone act via GABA pathways. Barbiturates are cross tolerance to alcohol and can ease withdrawal symptoms significantly. However, controlled studies have not provided sufficient data to demonstrate that these agents can prevent seizures or DT’s. Furthermore, barbiturates have a narrow therapeutic index, that is, the difference between the minimum doses required for a therapeutic effect and the dose at which the agents become toxic is small, as compared to BZDs and are not in common practice [3].
6. Baclofen
The advances in knowledge of neurobiology and neurochemistry have prompted the use of drugs in the treatment of alcohol withdrawal that act through "t GABA pathways", such as the Baclofen, which are GABA-B pathways (agonist). Addolorato et al., reported a case series with five patients in which a single 10-mg dose of Baclofen resulted in relief of severe withdrawal symptoms [68]. In a preliminary RCT by the first author in 2002, Baclofen also reduced craving in alcohol-dependent patients [69]. A study found that the efficacy of Baclofen in treatment of uncomplicated AWS was comparable to that of the “gold standard” diazepam, with significantly decreased CIWA-Ar scores [70].
7. Newer Drugs
Most of the recently tried drugs in AWS are being used only as adjuncts to BZDs. N-methyl-d-aspartate antagonist ketamine appears to reduce BZD requirements and is well tolerated at low doses [71]. However, there are not enough published data. Still, it is a promising new agent. Levetiracetam has also been tried but with negative results [72]. It did not significantly reduce the benzodiazepine requirements of patients with AWS. A review found that sodium oxybate, sodium salt of γ-hydroxybutyric acid, is a useful option for the treatment of alcohol withdrawal syndrome [73]. The salt is approved in Italy and Austria for the same. Dexmedetomidine is a drug which acts on the noradrenergic system and is currently used in the US in the treatment of AWS in emergency set up. It may reduce the need for BZD and is a promising and effective adjuvant treatment for AWS [74].
• Alcohol withdrawal syndrome with alcohol withdrawal seizures and/or delirium
The occurrence of seizures during the AWS is indicative of severe alcohol withdrawal, although the CIWA-Ar score may not correlate. All patients with AWS, with seizures in the current withdrawal period or past history of withdrawal seizure should be given prophylactic intravenous/intramuscular injection of 2mg lorazepam [75]. Lorazepam is considered more effective than diazepam in preventing seizure recurrence as lorazepam has consistent plasma level distribution unlike diazepam. These patients may require high doses of benzodiazepine (diazepam equivalents of about 20-60 mg) to prevent further seizures and to prevent the development of DT [51]. Patients with AW seizures should be ideally admitted and monitored for at least 36-48 h to watch for further seizures or DT [76]. Detailed neurological and medical examination, blood investigations and brain imaging are required and should be done, especially to rule out alternative causes.
Alcohol withdrawal delirium DT is a medical emergency and requires indoor treatment and monitoring. Detailed neurological and medical examination along with blood investigations should be done in all patients to rule out other common causes of delirium such as hypoglycemia, electrolyte imbalance, and head injury leading to subdural hemorrhage, septicemia and renal/liver failures. Brain imaging may be undertaken in suspected cases of neurological insult. The goal of management is to achieve a calm and awake state. The goal is best achieved through judicious use of BZDs. Intravenous or intramuscular lorazepam should be preferred and administered at frequent intervals with close monitoring. Lorazepam is more suitable in patients with hepatic disease, in the elderly where there is risk of over sedation and respiratory depression with diazepam. Initial doses of 10 mg equivalents of diazepam are given intravenously/intramuscularly and can be repeated every 15-30 minutes [51,77]. Some experts even advice and advocate use of loading doses of diazepam for management of DT. However, it is purely based on clinical experience as no clinical trials have been conducted in patients with DT. When light somnolence is achieved and the patient is relaxed, management may be shifted to oral/injectable symptom monitored schedule. Vitals monitoring is extremely important and should be done regularly.
• Adjunctive Supplements
Chronic alcohol use is associated with depletion of body stores of thiamine and magnesium. Chronic thiamine deficiency may result in classical triad of confusion, ataxia and ophthalmoplegia, better known as Wernicke’s Encephalopathy (WE). It results from cell damage of the mammillary body, thalamus and the hippocampal area. As the classical triad of WE rarely presents to clinic, it therefore goes undiagnosed in most of the patients. It may lead to a permanent severe amnestic syndrome known as Korsokoff’s encephalopathy. Parenteral thiamine is essential for prevention of WE. It is mandatory that thiamine should be given before intravenous administration of glucose in suspected cases of Wernicke’s encephalopathy, as glucose alone can precipitate the disorder in thiamine-deficient individuals. In patients without WE, an oral dose of thiamine 100 mg daily may be administered. But in all people with severe alcohol withdrawal, people with poor diet and signs of malnutrition, thiamine should be administered intramuscularly in the doses of 250 mg per day for 3—5 consecutive days [78,79].
Alcohol dependent patients may also be found deficient in vitamins and electrolytes, such as magnesium and niacin. Chronic alcohol use is associated with abnormal magnesium and niacin metabolism and absorption. Hence, the deficiencies need appropriate correction. Low serum magnesium level may lead to neuropathy and confusion. Multivitamins IV injections along with magnesium replacement should be given if symptoms are present or prophylactically in patients with severe AWS [80].
Conclusion
Alcohol Withdrawal Syndrome results in people who are dependent on alcohol and either stop drinking, or reduce the alcohol consumption. This results from a shift in the neurotransmitter levels in the brain, from GABA inhibition to glutaminergic stimulation. The symptoms are generally mild to moderate and resolve within a few days. However, severe forms of AWS may be associated with generalized seizures, hallucinations and delirium tremens, which can be fatal. AWS are best monitored by regular scale based assessments such as CIWA-Ar.
Outpatient withdrawal may be more appropriate for patients who are at low risk of developing severe withdrawal syndrome. Patients with moderate or severe alcohol withdrawal, medical complications and multiple failed attempts at abstinence may need close monitoring, in indoor setting. Oral benzodiazepines are the best studied and most effective drugs for preventing a severe alcohol withdrawal syndrome, particularly the risk of seizures and delirium. The management should be individualized with the help of rating scales and use of Symptom Triggered regime, which is proved to be more effective as compared to Fixed Tapering dose regime. Other important drugs used to manage AWS are anti-epileptics such as valproate, carbamazepine, gabapentin; adrenergic blockers such as Propanolol and clonidine; Baclofen; Barbiturates and recent drugs like levetiracetam, sodium oxybate and dexmedetomidine. For delirium tremens and withdrawal seizures, treatment with high-dose benzodiazepines (parenteral or oral) is recommended in ICU set up. Thiamine (B1) deficiency is commonly seen and serious complications in alcohol-dependent patients and hence, supplementation is widely recommended.
[1]. World BankWorld Development Report 1993: Investing in HealthNew YorkOxford University Press [Google Scholar]
[2]. Indian Council of Medical Reasearch Bulletin. Vol.38, No.1-3 January-March, 2008 [Google Scholar]
[3]. Saitz R, Introduction to alcohol withdrawalAlcohol Health Res World 1998 22:5-12. [Google Scholar]
[4]. Hasin DS, Grant B, Endicott J, The natural history of alcohol abuse: implications for definitions of alcohol use disordersAm J Psychiatry 1990 147(11):1537-41. [Google Scholar]
[5]. Kessler RC, Nelson CB, McGonagle KA, Liu J, Scwartz M, Blazer DG, Co-morbidity of DSM- III- R major depressive disorder in the general population: Results from the US National Co-morbidity SurveyBr J Psychiatry 1996 168:17-30. [Google Scholar]
[6]. Ray R, editor. The Extent, Pattern and Trends of Drug Abuse in India: National Survey. United Nations Office on Drugs and Crime, Regional Office for South Asia and Ministry of Social Justice and Empowerment, Government of India, New Delhi, June 2004 [Google Scholar]
[7]. DeWitte P, Pinto E, Ansseau M, Verbanck P, Alcohol and withdrawal: from animal research to clinical issuesNeurosci Biobehav Rev 2003 27(3):189-97. [Google Scholar]
[8]. Hall W, Zador D, The alcohol withdrawal syndromeLancet 1997 349(9069):1897-900. [Google Scholar]
[9]. Koob GF, Nestler EJ, The neurobiology of drug addictionJ Neuropsychiatry Clin Neurosci 1997 9(3):482-97. [Google Scholar]
[10]. Isbell H, Fraser HF, Wikler A, Belleville RE, Eisenman AJ, An experimental study of the etiology of “rum fits” and delirium tremensQuarterly Journal of Studies on Alcohol 1955 16:1-33. [Google Scholar]
[11]. Victor M, Adams RD, Effects of alcohol on the nervous systemRes Publ Assoc Res Nerv Ment Dis 1953 32:526-73. [Google Scholar]
[12]. Ng SKC, Hauser WA, Brust JCM, Susser M, Alcohol consumption and withdrawal in new-onset seizuresN Engl J Med 1988 319:666-73. [Google Scholar]
[13]. Dodd PR, Beckmann AM, Davidson MS, Wilce PA, Glutamate mediated transmission, alcohol, and alcoholismNeurochem Int 2000 37(5-6):509-33. [Google Scholar]
[14]. Kohl RR, Katner JS, Chernet E, McBride WJ, Ethanol and negative feedback regulation of mesolimbic dopamine release in ratsPsychopharmacology 1998 139(1-2):79-85. [Google Scholar]
[15]. Tsai G, Gastfriend DR, Coyle JT, The glutamatergic basis of human alcoholismAm J Psychiatry 1995 152(3):332-40. [Google Scholar]
[16]. Rogawski MA, Update on the neurobiology of alcohol withdrawal seizuresEpilepsy Curr 2005 5:225-30. [Google Scholar]
[17]. Miller NS, Gold MS, Management of withdrawal syndromes and relapse prevention in drug and alcohol dependenceAm Fam Physician 1998 58:139-46. [Google Scholar]
[18]. American Psychiatric AssociationDiagnostic and statistical manual of mental disorders: DSM-IV-TR 2000 Washington, DCAmerican Psychiatric Association [Google Scholar]
[19]. Bayard M, McIntyre J, Hill K, Woodside J Jr, Alcohol withdrawal syndromeAm Fam Physician 2004 69(6):1443-50. [Google Scholar]
[20]. Tovar R, Diagnosis and treatment of alcohol withdrawalClin Outcomes Manag 2011 18:361-70. [Google Scholar]
[21]. Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM, Assessment of alcohol withdrawal: the revised Clinical Institute Withdrawal Assessment for Alcohol scale (CIWA-Ar)Br J Addict 1989 84:1353-57. [Google Scholar]
[22]. Chabria SB, Inpatient management of alcohol withdrawal: A practical approachSigna Vitae 2008 3:24-29. [Google Scholar]
[23]. Manikant S, Tripathi BM, Chavan BS, Utility of CIWA-A in alcohol withdrawal assessmentIndian J Psychiatry 1992 34:347-50. [Google Scholar]
[24]. Mayo-Smith MF, American Society of Addiction Medicine Working Group on Pharmacological Management of Alcohol Withdrawal. Pharmacological management of alcohol withdrawal. A meta-analysis and evidence-based practice guidelineJAMA 1997 278(2):144-51. [Google Scholar]
[25]. Jaeger T, Lohr R, Pankratz VS, Symptom-triggered therapy for alcohol withdrawal syndrome in medical inpatientsMayo Clinic Procedure 2001 77:695-701. [Google Scholar]
[26]. Holbrook AM, Crowther R, Lotter A, Cheng C, King D, Diagnosis and management of acute alcohol withdrawalCMAJ 1999 160(5):675-80. [Google Scholar]
[27]. Rosenbloom A, Emerging treatment options in the alcohol withdrawal syndromeJ Clin Psychiatry 1988 49:28-32. [Google Scholar]
[28]. Kasser C, Geller A, Howell E, Wartenberg A, Detoxification: principles and protocolsAmerican Society of Addiction and Medicine. [cited 2010 Sept 7]Available from: http://www.asam.org/pub1/detoxification.htm [Google Scholar]
[29]. Abbott JA, Quinn D, Knox L, Ambulatory medical detoxification for alcoholAm J Drug Alcohol Abuse 1995 21(4):549-63. [Google Scholar]
[30]. Myrick H, Anton R, Treatment of Alcohol WithdrawalAlcohol Health Res World 1998 22(1):38-43. [Google Scholar]
[31]. Ballenger JC, Post RM, Kindling as a model for alcohol withdrawal syndromesBr J Psychiatry 1978 133:1-14. [Google Scholar]
[32]. Brown ME, Anton RF, Malcolm R, Ballenger JC, Alcohol detoxification and withdrawal seizures: Clinical support for a kindling hypothesisBiol Psychiatry 1988 23:507-14. [Google Scholar]
[33]. Rathlev NK, Ulrich AS, Delanty N, D’Onofrio G, Alcohol related seizuresJ Emerg Med 2006 31:157-63. [Google Scholar]
[34]. Bharadwaj B, Bernard M, Kattimani S, Rajkumar RP, Determinants of success of loading dose diazepam for alcohol withdrawal: a chart reviewJ Pharmacol Pharmacother 2012 3:270-72. [Google Scholar]
[35]. Hayashida M, Alterman AI, Mclellan T, Comparative effectiveness and costs of inpatient and outpatient detoxification of patients with mild-to-moderate alcohol withdrawal syndromeN Engl J Med 1989 320(6):358-65. [Google Scholar]
[36]. Porter R, The drinking man’s disease: the ‘pre-history’ of alcoholism in Georgian BritainBr J Addict 1985 80:385-96. [Google Scholar]
[37]. Faillace LA, Flamer RN, Imber SD, Ward RG, Giving alcohol to alcoholics: An evaluationQ J Stud Alcohol 1972 33:85-90. [Google Scholar]
[38]. Funderburk FR, Allen RP, Wagman AMI, Residual effects of ethanol and chlordiazepoxide treatments for alcohol withdrawalJ Nerv Ment Dis 1978 166:195-203. [Google Scholar]
[39]. Craft PP, Foil MB, Cunningham PRG, Patselas PC, Long-Snyder BM, Collier MS, Intravenous ethanol for alcohol detoxification in trauma patientsSouthern Medical Journal 1994 87:47-54. [Google Scholar]
[40]. Schatzberg AF, Cole JO, DeBattista C, Manual of Clinical Psychopharmacology 2003 4th edNew YorkAmerican Psychiatric Publishing Inc [Google Scholar]
[41]. Sellers EM, Naranjo CA, Harrison M, Devenyl P, Roach C, Sykora K, Diazepam loading dose: simplified treatment of alcohol withdrawalClin Pharmacol Ther 1983 34:822-26. [Google Scholar]
[42]. Adinoff B, Double-blind study of alprazolam, diazepam, clonidine, and placebo in the alcohol withdrawal syndrome: preliminary findingsAlcohol Clin Exp Res 1994 18:873-78. [Google Scholar]
[43]. Ozdemir V, Bremner KE, Naranjo CA, Treatment of alcohol withdrawal syndrome. Trends in Clinical PracticeAnn Med 1993 26:101-05. [Google Scholar]
[44]. Nutt D, Adinoff B, Linnoila M, Benzodiazepines in the treatment of alcoholism. In: Galanter M, editorRecent Development in Alcoholism 1989 Vol. 8New YorkPlenum Press:283-313. [Google Scholar]
[45]. Holbrook AM, Crowther R, Lotter A, Cheng C, King D, Meta-analysis of benzodiazepine use in the treatment of acute alcohol withdrawalCMAJ 1999 160:649-55. [Google Scholar]
[46]. Saitz R, Mayo-Smith MF, Roberts MS, Redmond HA, Bernard DR, Calkins DR, Individualized treatment for alcohol withdrawal. a randomized double-blind controlled trialJAMA 1994 272:519-23. [Google Scholar]
[47]. Daeppen JB, Gache P, Landry U, Sekera E, Schweizer V, Gloor S, Symptom-triggered vs fixed-schedule doses of benzodiazepine for alcohol withdrawal: a randomized treatment trialArch Intern Med 2002 162:1117-21. [Google Scholar]
[48]. Sullivan JT, Swift RM, Lewis DC, Benzodiazepine requirements during alcohol withdrawal syndrome: clinical implications of using a standardized withdrawal scaleJ Clin Psychopharmacol 1991 11:291-95. [Google Scholar]
[49]. Manikant S, Tripathi BM, Chavan BS, Loading dose diazepam therapy for alcohol withdrawal stateIndian J Med Res 1993 98:170-73. [Google Scholar]
[50]. Sellers EM, Sandor P, Giles HG, Khouw V, Greenblatt DJ, Diazepam pharmacokinetics after intravenous administration in alcohol withdrawalBr J Clin Pharmacol 1983 15:125-27. [Google Scholar]
[51]. Saitz R, O’Malley SS, Pharmacotherapies of alcohol abuse: Withdrawal and treatmentMed Clin North Am 1997 81(4):881-907. [Google Scholar]
[52]. Reoux JP, Miller K, Routine hospital alcohol detoxification practice compared with symptom triggered management with an Objective Withdrawal Scale (CIWA-Ar)Am J Addict 2000 9:135-44. [Google Scholar]
[53]. Day EJ, Patel JV, Georgiou GA, A pilot study to evaluate a symptom-triggered front-loading detoxification technique for alcohol dependencePsychiatr Bull 2004 28(11):407-10. [Google Scholar]
[54]. Cassidy EM, O’Sullivan I, Bradshaw P, Symptom-triggered benzodiazepine therapy for alcohol withdrawal syndrome in the emergency department: a comparison with the standard fixed dose benzodiazepine regimenEmerg Med J 2012 29:802-04. [Google Scholar]
[55]. Lange-Asschenfeldt C, Müller MJ, Szegedi A, Anghelescu I, Klawe C, Wetzel H, Symptom-Triggered versus Standard Chlormethiazole Treatment of Inpatient Alcohol Withdrawal: Clinical Implications from a Chart AnalysisEur Addict Res 2003 9:1-7. [Google Scholar]
[56]. Sachdeva A, Chandra M, Deshpande SN, A Comparative Study of Fixed Tapering Dose Regimen versus Symptom-triggered Regimen of Lorazepam for Alcohol DetoxificationAlcohol Alcohol 2014 49(3):287-91. [Google Scholar]
[57]. Malcolm R, Ballenger JC, Sturgis ET, Anton R, Double-blind controlled trial comparing carbamazepine to oxazepam treatment of alcohol withdrawalAm J Psychiatry 1989 146:617-21. [Google Scholar]
[58]. Malcolm R, Myrick H, Roberts J, Wang W, Anton RF, Ballenger JC, The effects of carbamazepine and lorazepam on single versus multiple previous alcohol withdrawals in an outpatient randomized trialJ Gen Intern Med 2002 17:349-55. [Google Scholar]
[59]. Stuppaeck CH, Pycha R, Miller C, Whitworth AB, Oberbauer H, Fleischhacker WW, Carbamazepine versus oxazepam in the treatment of alcohol withdrawal: a double-blind studyAlcohol Alcohol 1992 27:153-58. [Google Scholar]
[60]. Asplund CA, Aaronson JW, Aaronson HE, Three Regimens for Alcohol Withdrawal and DetoxificationJ Fam Pract 2004 53(7):545-54. [Google Scholar]
[61]. Reoux JP, Saxon AJ, Malte CA, Baer JS, Sloan KL, Divalproex sodium in alcohol withdrawal: a randomized double-blind placebo-controlled clinical trialAlcohol Clin Exp Res 2001 25:1324-29. [Google Scholar]
[62]. Malcolm R, Myrick H, Brady KT, Ballenger JC, Update on anticonvulsants for the treatment of alcohol withdrawalAm J Addict 2001 10(Suppl):16-23. [Google Scholar]
[63]. Myrick H, Malcolm R, Brady KT, Gabapentin treatment of alcohol withdrawalAm J Psychiatry 1998 155:1632 [Google Scholar]
[64]. Bozikas V, Petrikis P, Gamvrula K, Savvidou I, Karavatos A, Treatment of alcohol withdrawal with gabapentinProg Neuropsychopharmacol Biol Psychiatry 2002 26:197-99. [Google Scholar]
[65]. Chourishi A, Raichandani OP, Chandraker S, Chourishi S, A comparative study of efficacy and tolerability of lorazepam and gabapentin in the treatment of alcohol withdrawal syndromeIJPSR 2010 3(2):80-84. [Google Scholar]
[66]. Stuppaeck CH, Deisenhammer EA, Kurz M, Whitworth AB, Hinterhuber H, The irreversible gamma-aminobutyrate transaminase inhibitor vigabatrin in the treatment of the alcohol withdrawal syndromeAlcohol Alcohol 1996 31:109-11. [Google Scholar]
[67]. Anton RF, Becker HC, Pharmacology and pathophysiology of alcohol withdrawal. In: Kranzler HR, editorHandbook of Experimental Pharmacology: Volume 114. The Pharmacology of Alcohol Abuse 1995 New YorkSpringer-Verlag:315-67. [Google Scholar]
[68]. Addolorato G, Caputo F, Capristo E, Janiri L, Bernardi M, Agabio R, Rapid suppression of alcohol withdrawal syndrome by baclofenAm J Med 2002 112:226-29. [Google Scholar]
[69]. Addolorato G, Caputo F, Capristo E, Domenicali M, Bernardi M, Janiri L, Baclofen efficacy in reducing alcohol craving and intake: a preliminary double-blind randomized controlled studyAlcohol Alcohol 2002 37:504-08. [Google Scholar]
[70]. Addolorato G, Leggio L, Abenavoli L, Agabio R, Caputo F, Capristo E, Baclofen in the treatment of alcohol withdrawal syndrome: a comparative study vs diazepamAm J Med 2006 119(3):276.e13-18. [Google Scholar]
[71]. Wong A, Benedict NJ, Armahizer MJ, Kane-Gill SL, Evaluation of adjunctive ketamine to benzodiazepines for management of alcohol withdrawal syndromeAnn Pharmacother 2015 49(1):14-19. [Google Scholar]
[72]. Youland KM, Miller RF, Mahoney LJ, Borgert AJ, Gundrum JD, Levetiracetam as adjunctive therapy for acute alcohol withdrawal syndrome in hospitalized patientsJ Clin Psychopharmacol 2014 34(6):704-08. [Google Scholar]
[73]. Keating GM, Sodium oxybate: a review of its use in alcohol withdrawal syndrome and in the maintenance of abstinence inalcohol dependenceClin Drug Investig 2014 34(1):63-80. [Google Scholar]
[74]. Muzyk AJ, Kerns S, Brudney S, Gagliardi JP, Dexmedetomidine for the treatment of alcohol withdrawal syndrome: rationale and current status of researchCNS Drugs 2013 27(11):913-20. [Google Scholar]
[75]. D’Onofrio G, Rathlev NK, Ulrich AS, Fish SS, Freedland ES, Lorazepam for the prevention of recurrent seizures related to alcoholN Engl J Med 1999 340:915-19. [Google Scholar]
[76]. Eyer F, Schuster T, Felgenhauer N, Pfab R, Strubel T, Saugel B, Risk assessment of moderate to severe alcohol withdrawal – Predictors for seizures and delirium tremens in the course of withdrawalAlcohol Alcohol 2011 46:427-33. [Google Scholar]
[77]. Lal R, Pharmacotherapy of substance use disorders. In: Lal R, editorSubstance Use Disorders: Manual for Physicians 2005 New DelhiNational Drug Dependence Treatment Center, All India Institute of Medical Sciences [Google Scholar]
[78]. Cook CCH, Hallwood PM, Thomson AD, B-vitamin deficiency and neuro-psychiatric syndromes in alcohol misuseAlcohol Alcohol Suppl 1998 33:317-36. [Google Scholar]
[79]. Cook CC, Prevention and treatment of Wernicke-Korsakoff SyndromeAlcohol Alcohol Suppl 2000 35:19-20. [Google Scholar]
[80]. Sullivan JF, Lankford HG, Swartz MJ, Farrell C, Magnesium metabolism in alcoholismAm J Clin Nutr 1963 13:297-303. [Google Scholar]