Hypokalaemic Periodic Paralysis (HPP) represents a group of disorders presenting with acute flaccid paralysis, with a documented hypokalemia (K+< 3.5mEq/L) during the episode and recovery following treatment [1]. It closely resembles Guillain-Barré Syndrome (GBS). HPP is completely reversible with appropriate therapy in patients presenting with acute flaccid paralysis. However, if it is not recognized early, it can lead to life threatening complications such as arrhythmias. It can also be easily misdiagnosed as GBS, which leads to an incorrect management, which can lead to potentially life threatening sequelae. According to the available literature on HPP, the predominant aetiology of HPP varies between different populations. For instance, in India, a case series study showed that HPP was predominant secondary to Renal Tubular Acidosis (RTA) and primary hyperaldosteronism [1]. In contrast, studies done in a Caucasian population found Familial Periodic Paralysis (FPP) as the commonest aetiology of HPP while another study from the Far East found Thyrotoxic Periodic Paralysis (TPP) as the commonest cause [2,3].
To study the patients with HPP and compare our results with the aforementioned studies.
Materials and Methods
All the patients in the age group 18-65 years who were diagnosed with HPP (K+< 3.5mEq/L) were included in the study. The exclusion criteria included ascending paralysis due to any other diagnoses or if any patient did not consent to take part in this study. A total of 23 patients (19 Males and 4 Females) diagnosed with HPP during the time period of September 2011 to September 2014 were assessed.
A detailed history was obtained from the patients that included age, duration of illness, history of renal stones, bony pains, dry mouth, dry eyes, fractures, family history of HPP (if positive, with a special mention of the time of presentation), relation to food intake, strenuous exercise and mode of progression. A routine physical examination was performed with an emphasis on blood pressure, parotid and lacrimal gland examination and a detailed neurological examination. All patients were investigated for the following laboratory parameters – random blood sugar, renal function tests (serum urea, creatinine), serum electrolytes (sodium, potassium, chloride, and magnesium), thyroid function tests (Free T3, Free T4, and TSH), arterial blood gas analysis and urine pH.
Based on the results of the laboratory investigations, further work up was done accordingly. Patients with Thyrotoxic Periodic Paralysis (TPP) were evaluated with a radionucleotide thyroid scan [4]. Patients with hypokalaemia secondary to loss of K+ from the GI tract were investigated for underlying GI pathology by colonoscopy and serological investigation for Anti-Sacchyromyces Cerevisiae Antibodies (ASCA). Patients, whose baseline workup was suggestive of primary hyperaldosteronism, were investigated with an Aldosterone/Renin ratio and contrast enhanced CT Abdomen (CECT) [5]. Patients who had hypokalaemia secondary to renal causes were investigated accordingly [6] (for e.g., patients with Hyperchloremic Metabolic Acidosis with a normal anion gap and an absence of gastrointestinal losses, a fasting urine pH >5.5 was taken to denote renal tubular acidosis). [Table/Fig-1] outlines the basic classification of HPP used in this study while [Table/Fig-2] shows classification of renal aetiologies of HPP based on arterial blood gas analysis and urine pH.
| Primary HPP | Secondary HPP |
|---|
| Single Gene Mutation in the Ca2+, Na+ or K+ Channels [7], | 1. Thyrotoxic Periodic Paralysis [8] |
| 2. Gastrointestinal |
| 3. Renal |
| 4. Diuretic/Drug use |
Aetiologies of HPP due to renal causes [9]
| Metabolic Alkalosis | Metabolic Acidosis |
|---|
| Alkaline Urine | Acidic urine | Alkaline Urine |
|---|
| Hypertension and raised serum Na+Primary HyperaldosteronismLiddle’s Syndrome | Primary Renal Tubular Acidosis | Recovery from Renal Tubular acidosis |
| NormotensiveBartter SyndromeGitelman SyndromeJ-G apparatus hypertrophy with severe hyperaldosteronism | | Recovery from Diabetic Ketoacidosis |
Results
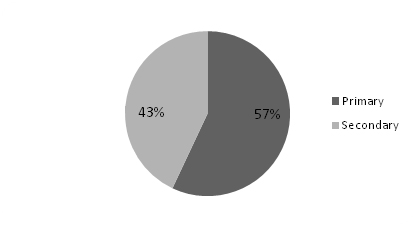
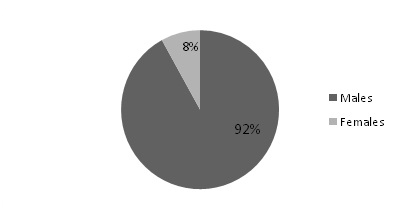
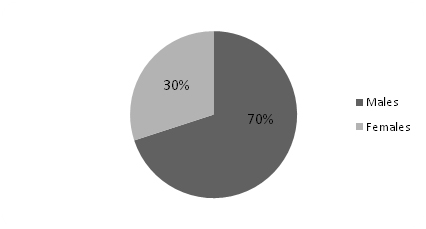
Of the 23 patients diagnosed with HPP, 13(57%) had primary HPP and 10(43%) had secondary HPP [Table/Fig-3]. Secondary HPP presented at a mean age of 38 years, while primary HPP was seen in younger group with a mean of 28 years. In both the groups, men were predominantly affected [Table/Fig-4,5]. Primary HPP presented with a shorter mean duration of symptoms of 18 hours while secondary HPP usually presented much later with mean duration of symptoms of 60 hours. Ascending paralysis was a major presenting feature that was present in nearly 80% of patients in both groups. This feature can easily mislead be confused with GBS. Hence, one should rule out all the other possibilities of ascending paralysis, including HPP before starting treatment for GBS. Early morning symptoms were seen in a majority (77%) of the patients with primary HPP whereas only a fifth (20%) of patients with secondary HPP had the same. Similarly, weakness associated with a carbohydrate meal or strenuous exercise was seen in 46% of patients with primary HPP and only 10% of patients with secondary HPP. Thus, features of early morning paralysis and history of weakness related to a meal or strenuous exercise seem to have better sensitivity when ruling out GBS. Nearly half of the patients (48%) were referred to our hospital with a diagnosis of GBS. Results of both the groups (primary and secondary) are compared in [Table/Fig-6].
Gender distribution in Primary HPP
Gender distribution in Secondary HPP
Comparing our results of primary and secondary HPP
| Primary HPP (57%) (N = 13) | Secondary HPP (43%) (N = 10) |
|---|
| Mean Age (years) | 28 (18-37) | 38.7 (22-56) |
| Males | 12(92%) | 7(70%) |
| Females | 1(8%) | 3(30%) |
| Mean duration of symptoms (hours) | 18 (4-24) | 60 (6-120) |
| Patients presenting with Ascending Paralysis | 10(77%) | 8(80%) |
| Patients presenting with symptoms in early morning | 10(77%) | 2(20%) |
| Patients with history of weakness in relation to a carbohydrate meal or strenuous exercise | 6(46%) | 1(10%) |
Thyrotoxic Periodic Paralysis (TPP) was the leading cause of secondary HPP, underscoring the importance of thyroid function tests in patients presenting with ascending paralysis. Gastrointestinal and renal pathologies made up for 30% and 20% of cases of secondary HPP respectively. In [Table/Fig-7] the causes of secondary HPP from our study are listed.
Aetiologies of secondary HPP
| Thyrotoxic Periodic Paralysis | 5(50%) |
| Infective Diarrhoea | 2(20%) |
| Crohn’s disease | 1(10%) |
| Conn’s Syndrome | 1(10%) |
| RTA Type I | 1(10%) |
Out of 5 patients with TPP, 2 had Grave’s disease and were treated with radio-ablation. The other 3 responded well to Anti-thyroid drugs and beta-blockers. Out of 3 patients who presented with HPP due to GI loss, 1 patient had hypoalbuminemia and persistent weight loss. Further work up by colonoscopy revealed Crohn’s disease and was also ASCA positive. Hence the patient was referred to gastroenterologists for further management. One patient presented with metabolic alkalosis, hypertension, alkaline urine and (Aldosterone/Renin) >20. Liddle’s syndrome was also considered as a differential because it has been shown to cause HPP [10]. But CECT of the abdomen revealed adrenal adenoma, which was successfully excised [11].
Discussion
In a study of 31 patients by Rao N et al., the secondary causes for HPP were analysed, where 13 patients were diagnosed with renal tubular acidosis, 13 with primary hyperaldosteronism, 2 with thyrotoxic periodic paralysis, 2 sporadic periodic paralysis, and 1 with Gitelman syndrome [1]. However, in the present study, TPP was found in 50% of the cases.
The patients of TPP usually have the classical symptoms of hyperthyroidism such as weight loss, insomnia, tremors, increased appetite, palpitations and heat intolerance. After these symptoms set in, patients experience intermittent episodes of weakness usually in the shoulder and hip joints muscles. They can also complain of weakness following a high carbohydrate meal or strenuous exercises [12]. Early diagnosis and prompt treatment of TPP can prevent life-threatening complications of this treatable and curable disorder [9]. Manoukian MA et al., studied 24 episodes of TPP in 19 patients and found that hypokalemia was present in all 24 initial episodes [4].
A study in North eastern part of India included 56 patients of which 24 had hypokalaemic paralysis due to secondary cause, which included 4 with distal RTA, 4 with Gitelman syndrome, 3 with TPP, 2 each with hypothyroidism, gastroenteritis and Liddle’s syndrome, 1 primary hyperaldosteronism, 3 with alcoholism and 1 with dengue fever [13].
In a retrospective study by Kalita J et al., among 52 patients with HPP; nine had TPP and 27 had IHPP (idiopathic HPP). The biochemical analysis showed that the serum potassium was significantly lower in TPP compared to IHPP [14].
The main site of potassium ion absorption in the body is the small intestine. Hence, loss of potassium ions through the gastrointestinal tract can be seen in cases of acute gastroenteritis commonly. It can also present in patients with short bowel syndrome, gastrointestinal fistula and Crohn’s disease. Any pathology that interferes with pH buffering mechanism of the kidneys can result in electrolyte disturbances leading to HPP. Patients suffering from such renal pathology can present with symptoms of acidosis such as hyperventilation, kidney stones etc or symptoms of alkalosis such as hypoventilation, drowsiness etc. In both these cases they can have decreased urine output, hypoalbuminemia, hypertension, flank or back pain and arrhythmia [15].
In a study of 40 patients of HPP, the common precipitating factors were found as diarrhoea, fever, strenuous activity, following dextrose administration, in patients with diabetic ketoacidosis [16]. Although in majority of the cases the precipitating factors could not be identified by the researchers. Most of the patients had recurrent episodes of paralysis and the common secondary cause for hypokalaemic paralysis was renal tubular acidosis. About 10% of patients were diagnosed with RTA in the present study.
Conclusion
While primary HPP was more common, secondary HPP still contributed to 43% of the cases, which is a significant proportion of patients with potentially treatable underlying pathology. This underscores the importance of a complete workup including renal function tests, serum electrolytes, thyroid function tests, ABG and urine pH to diagnose the cause of HPP. A significant conclusion drawn is that almost half the patients were misdiagnosed and mismanaged as GBS before being referred to a higher centre. This calls for spreading awareness regarding HPP, both primary and secondary, as a differential while treating patients with ascending paralysis.