Introduction
Periodontal disease is the common chronic bacterial infection of the supporting structures of the teeth. Several evidences has been put forth linking periodontal disease with various systemic diseases. Maternal periodontal disease has been studied in association with adverse pregnancy outcomes, including preterm labour, low birth weight, pre-eclampsia and miscarriages [1]. Preterm is one of the common causes for neonatal mortality and morbidity [2]. Preterm birth is defined as childbirth occurring at less than 37 completed weeks or 259 days of gestation (WHO 1993). It affects 20-50% of pregnant women globally [3]. About 15 million preterm births occur and 1 million children die every year due to its complications [2]. Different risk factors play a role in the higher rates of preterm birth which can be considered primary if they are present before the pregnancy or secondary if they develop during the course of the pregnancy [4] [Table/Fig-1].
Primary and secondary predictors for preterm birth [4]
| Primary predictors | Secondary predictors |
|---|
| • Black race | • No or inadequate prenatal care |
| • Young mother | • In vitro fertilization |
| • Domestic violence | • Low maternal weight gain late in pregnancy |
| • Low socio-economic status | • Iron-deficiency anemia |
| • Stress or depression | • Pre-eclampsia |
| • Cigarette smoking | • Elevated fetal fibronectin, a-fetoprotein, alkaline phosphatase, or granulocyte colony-stimulating factor |
| • Cocaine or heroin use | • Early contractions |
| • Low body mass index | • Vaginal bleeding in first or second trimester |
| • Low maternal weight gain before pregnancy | • Short cervical length |
| • Previous preterm birth or second trimester pregnancy loss | • Bacterial vaginosis, especially early in pregnancy |
| • Previous induced abortion | • Chorioamnionitis |
| • Family history/inflammatory gene polymorphisms | • Placental abruption |
| • Chronic lung disease | • Placenta previa |
| • Chronic hypertension | • Hydramniosis |
| • Diabetes | • Pre-eclampsia |
| • Renal disease | • Multiple foetuses |
The growing concept that infection remote from the feto–placental unit may influence preterm delivery of low birth-weight infants has led to an awareness of the potential role of chronic bacterial infections in the body. Periodontal diseases are extensively studied to assess the systemic influence of periodontal pathogens on adverse pregnancy outcomes. The pattern recognition receptors such as toll like receptors are implicated in the pathogenesis of preterm birth as a consequence of periodontal diseases. This article reviews on the plausible association of toll like receptors, periodontal diseases and preterm birth.
Pathogenesis of preterm labour
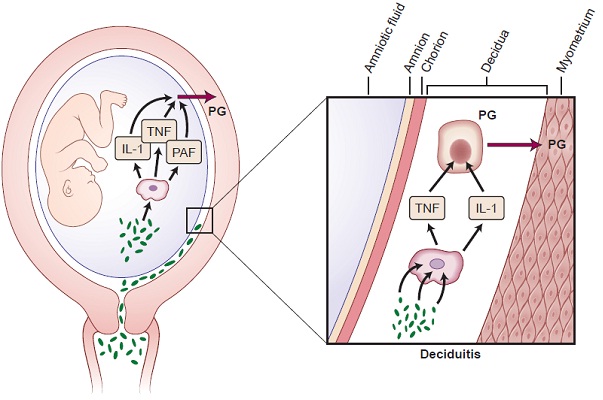
Multiple pathologic processes can lead to activation of the common pathway of parturition. They include uterine over distention, ischemia and haemorrhage, cervical diseases, abnormal allograft rejection, allergic reaction, endocrine disorder and infection [5]. Among these, infection is a frequent and important mechanism causing preterm delivery. Various infections implicated in the aetiology of preterm delivery are intrauterine infection and extrauterine maternal infections (malaria, pyelonephritis, pneumonia and periodontal disease). Pregnant women with intra-amniotic infection or inflammation are at risk for subsequent spontaneous preterm birth [6,7]. Bacterial products such as endotoxin have also been detected in the amniotic cavity of women with preterm labour and Preterm premature rupture of membrane (pPROM). Systemic dissemination of endotoxin leads to activation of a wide spectrum of cytokines such IL-1β, TNF-α, IL-1,6,8,10,16,18 [8,9]. These cytokines in turn regulates the secretion of mediators like platelet activating factors and prostaglandins which has been implicated in infection-induced preterm delivery [9].
Role of periodontal disease on preterm birth
Periodontal disease involves tissue destruction, characterized by the formation of periodontal pockets that act as reservoirs for bacterial colonization. Current evidence demonstrated a specific group of gram negative anaerobic bacteria including Aggregatibacter Actinomycetemcomitans, Tannerella forsythia, Campylobacter rectus, Fusobacterium nucleatum, Prevotella intermedia, Porphyromonas gingivalis as the key microorganisms involved in periodontitis [10]. Based on biological plausibility, it is believed that periodontitis can contribute to adverse pregnancy outcomes through colonization of periodontal bacteria in the uterine cavity and elicit an inflammatory cascade that leads to preterm labour. It is also presumed that the local cytokines secreted in response to the periodontal pathogens can also disseminate systemically and precipitate parturition [11]. According to this mechanism, most bacteria associated with progressive periodontitis are anaerobes, which would rarely survive the aerobic environment to enter the bloodstream [12]. Hence, it is generally accepted that the role of periodontal infection as a possible risk factor for PLBW more likely involves translocation of bacterial products (specifically LPS) or inflammatory mediators (specifically IL-1, IL-6, TNF-α and PGE2) rather than bacteraemia and translocation of the bacteria themselves [12] [Table/Fig-2].
Cellular and biochemical mechanisms involved in initiation of preterm labour in cases of intrauterine infection
Toll Like Receptors
The pattern recognition receptors (PRRs) are the receptors of innate immune system that evolved to recognize Microbe associated molecular patterns (MAMPs). Toll-like receptors (TLRs) are one of the most widely studied PRRs that play a role in the activation of cascade of inflammatory cytokines in the pathogenesis of preterm birth. In 1985, Toll gene was identified in Drosophila by Christine nusslein-Volhard and found to be responsible for dorso-ventral polarity during embryonic development [13]. The association of TLRs with innate immunity was recognized with the discovery of mouse TLR4, which acts as a receptor of bacterial lipopolysacchride. To date, eleven mammalian TLR have been identified, and these are believed to detect a discrete collection of molecules of microbial origin, and to signal the presence of infections.
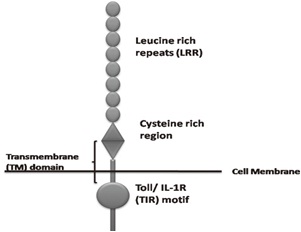
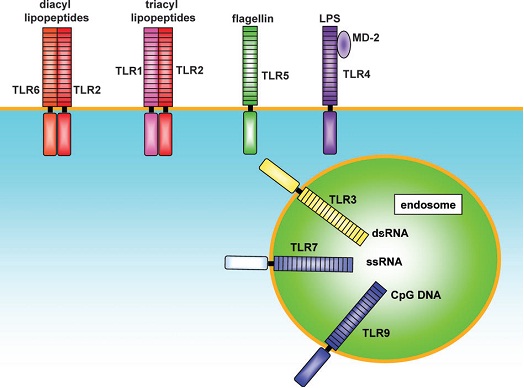
Toll like receptors belong to the “Interleukin-1 Receptor/Toll like Receptor superfamily” with a common Toll-IL-1receptor (TIR) domain [14] [Table/Fig-3]. Each subtypes of TLR recognize and bind to specific ligand molecules from the microbes. TLR2 form heterodimers with TLR1 and TLR6 and recognize triacyl lipopeptides and diacyl lipopeptides, lipoteichoic acid, peptidoglycan respectively. TLR4 is an essential receptor for LPS recognition and also involved in recognition of endogenous ligands, such as heat shock proteins (HSP60 and HSP70). TLR5 recognize flagellin through close physical interaction. TLR3, 7 and 9 are involved in the recognition of nucleic acid-like structure of the virus [Table/Fig-4].
Structure of a toll like receptor
After ligand binding, TLRs/IL-1Rs dimerize and undergo the conformational change required for the recruitment of downstream signalling molecules. These include the adaptor molecule myeloid differentiation primary-response protein 88 (MyD88), IL-1R-associated kinases (IRAKs), transforming growth factor-β (TGF-β)-activated kinase (TAK1), TAK1-binding protein 1 (TAB1), TAB2 and tumor-necrosis factor (TNF)-receptor-associated factor 6 (TRAF6) [15].
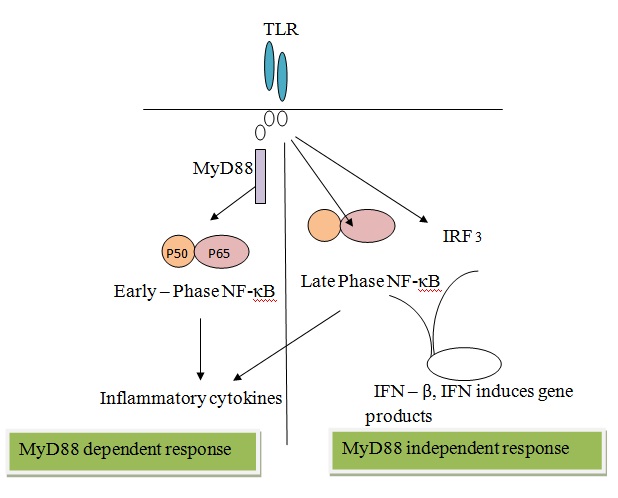
TLR stimulation activates two pathways: the MyD88-dependent and MyD88-independent pathways [16]. The MyD88-dependent pathway involves the activation of nuclear factor-κB (NF-κB), which leads to the production of inflammatory cytokines. Whereas, MyD88-independent pathway leads to the late phase of NF-κB activation through interferon-regulatory factor (IRF3) resulting in production of IFN-β [Table/Fig-5].
Outline of tlr signalling pathway
Toll Like Receptor As A Key Marker
These TLRs are expressed on several cells including monocytes, macrophages and leucocytes. On ligation of the PAMPs to the TLRs, they activate the production of cytokine through NF-κB pathway. Interestingly both periodontal diseases and adverse pregnancy outcomes have been studied in association with the expression of TLR and its effect on disease progression [17–29].
Chronic TLR stimulation in periodontal tissues by bacterial PAMPs can lead to excessive production of pro-inflammatory mediators, resulting in tissue destruction and systemic dissemination of the inflammatory mediators. Consistently, the expressions of the TLR in gingival and periodontal tissues have been reported both in healthy and diseased [19–22]. Gingival epithelial cells express TLR1, TLR2, TLR3, TLR4, TLR5, TLR6 (Kusumoto et al.,) [17], TLR7 (Uehara et al.,) [18], TLR9 (Kusumoto et al.,) [17] and TLR10 (Kinane DF et al.,) [19] and are therefore able to respond to hundreds of different microorganisms [26]. Elevated levels of TLR-2 and 4 expression in periodontal tissues of chronic periodontitis patients have been reported (Sarah SM et al.,) [20]. Tabeta et al., demonstrated that P.gingivalis LPS induced TLR-4 expression in human gingival fibroblasts [21].
Similarly, TLRs are expressed in the female reproductive tract and they play role in maintaining homeostasis throughout pregnancy [23]. TLR-2, 4 expression in is trophoblast cells of placenta and its implication in foetal infection has been previously reported [24]. Activation of TLRs on trophoblast cells influences immune cell recruitment, cytokine secretion, and decidual responses to invading pathogens. TLR signalling pathways were suggested as novel mechanism for the feto-placental unit to interact with microorganisms by the Holmlund et al., based on his study on human placental expression of the TLR-2 and TLR-4 in response to zymosan and LPS [22]. Among the TLRs, TLR1, TLR2, TLR4 and TLR6 may contribute to several pregnancy pathologies associated with pre-eclampsia, intrauterine growth restriction and preterm labour/birth [25,26].
Similarly, Abrahams et al., evaluated the expression of TLR1-10 in fetal membranes after exposure to pathogens associated with intra-amniotic infection and PTB. Normal human term fetal membrane explants were exposed to various bacteria [27]. After 24h RNA was extracted and quantitative RT-PCR performed for TLRs 1–10. Treatment of fetal membranes with Mycoplasma hominis increased expression of TLR4, TLR6, and TLR8 mRNA. Ureaplasma parvum upregulated TLR8 mRNA and Porphyromonas gingivalis significantly increased fetal membrane TLR7 expression. In contrast, treatment with Gram-negative Escherichia coli (and its cell wall component LPS) downregulated TLR10mRNA. No effect was detected for Ureaplasma urealyticum, Gardnerella vaginalis, or Group B Streptococcus. These results showed the specificity in the TLR response to difficult PAMPs from microorganisms and its association with infection-associated pregnancy complications, such as pPROM and PTB [27].
Consensus on the TLR Related Link Between Preterm Birth and Periodontal Disease
Arce RM et al., demonstrated increased TLR4 expression in a murine pregnancy model in response to oral infection with the human periodontal pathogens Campylobacter rectus and Porphyromonas gingivalis [28]. Chaparro A et al., proved an association in the placental tissue between the presence of T. denticola and P. gingivalis and preeclampsia leading to preterm labour and he also observed increased TLR-2 expression [29]. The above studies demonstrated that pathogens associated with maternal periodontitis can stimulate placental inflammation involving TLR 2 and 4 activation leading to down-stream of molecular signalling that causes production of various mediators of preterm births.
Recently, both maternal and fetal polymorphisms of TLRs which have been studied in association with preterm birth (PB), have also been studied in association with periodontitis. Two recently identified mutations of the Toll-like receptor-4 gene (Asp299Gly and Thr399Ile) were reported to attenuate human responsiveness to lipopolysaccharide both, invitro and invivo [30]. The two polymorphisms of the TLR-4 gene (Asp299Gly and Thr399Ile) and TLR-2 gene (Arg677Trp and Arg753Gln) are implicated in the pathogenesis of chronic periodontitis [30]. Also, polymorphisms in TLR4 gene also have been implicated in preterm birth [31]. This suggests a possible link between periodontal diseases and PB, based on an individual’s genetically determined predisposition to mount a hyperinflammatory response [32]. Although no definitive relationship has been established and other explanations for the correlation might be offered, a model can be contemplated explaining the periodontal disease as possible aetiology for preterm birth [Table/Fig-6].
The biological plausibility of periodontal disease and preterm birth
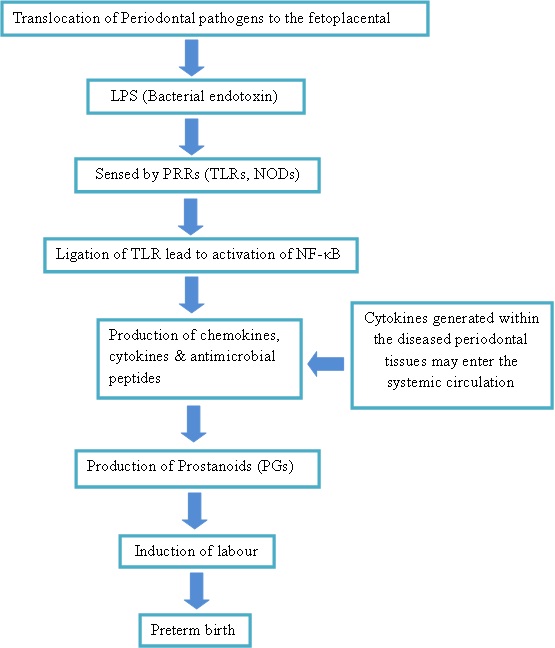
Clinical Significance
The effect of treatment of periodontal diseases did not prove to reduce the incidence of preterm birth. Various systematic reviews and meta-analysis concur that treatment of periodontitis with scaling and root planning during gestational period did not affect the outcome of pregnancy [33–35]. However, by providing appropriate treatment prior to conception or during the early pregnancy period can alter the outcome of pregnancy. Although the biological plausibility proves that there is an association of periodontal disease affecting the pregnancy outcomes, current evidence from RCTs does not support the provision of periodontal therapy to improve pregnancy outcomes. Hence, other therapeutic modalities should be studied to reduce the risk of preterm births in pregnant women with exposure to periodontal pathogens.
TLR Blockade in Prevention of Preterm Birth
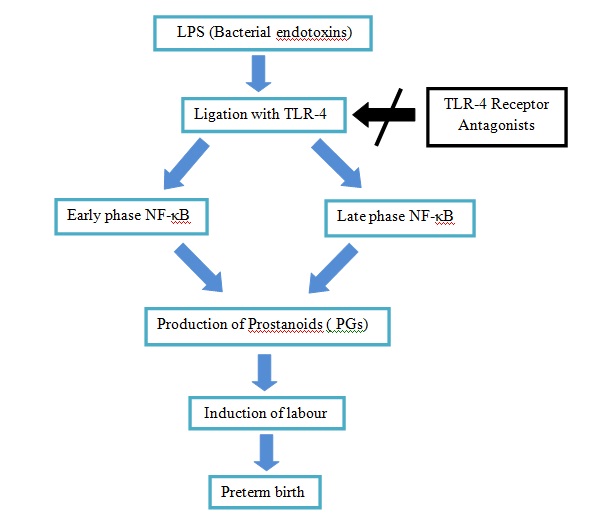
A preliminary study in blockade of LPS - induced immune responses by a Toll-like receptor 4 antagonist (TLR4A) which would prevent elevations in amniotic fluid (AF) cytokines, prostaglandins, and uterine contractility was conducted in rhesus monkeys. They demonstrated that compared to saline controls, LPS induced significant elevations in AF IL-8, TNF-α, PGE2, PGF2α, and uterine contractility. In contrast, TLR4A pre-treatment inhibited LPS-induced uterine activity and was associated with significantly lower AF IL-8, TNF-α, PGE2, and PGF2α versus LPS alone [36].
Hence, the role of TLR in pathogenesis raises the possibility that TLR blockade may become a critical component in treating preterm labour induced by gram-negative bacteria. The blocking of TLR, the earliest step in the immune response may down-regulate cytokine and prostaglandin synthesis which is the cornerstone for infection-induced preterm labour. Further prospective studies are required to evaluate the clinical effect of this treatment modality on reducing the risk of preterm births [Table/Fig-7].
TLR-4 receptor antagonists (ra) can block activation of TLR -4 and subsequent don stream of signalling molecules to prevent preterm labour
Conclusion
The association of maternal periodontitis and preterm birth has been extensively studied in the past. Nevertheless, intervention studies failed to demonstrate reduction in the risk for development of preterm birth in response to the non-surgical periodontal treatment. Hence understanding of biochemical and cellular mechanisms of pathogenesis of disease will open new insights in pre-treating women blocking TLR and immune response at an earlier level. Also, maternal immunization may help provide protection against foetal exposures during pregnancy. In future, human intervention studies have to be conducted to ensure the efficacy of this new modality.
[1]. Offenbacher S, Lieff S, Boggess KA, Murtha AP, Maternal periodontitis and prematurity. Part I: Obstetric outcome of prematurity and growth restrictionAnn Periodontol 2001 6(1):164-74. [Google Scholar]
[2]. Liu L, Johnson H, Cousens S, Global, regional and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000The Lancet 2012 379:2151-61. [Google Scholar]
[3]. WHO fact sheet No: 363, Updated November 2014. World health Organization [Google Scholar]
[4]. Michalowicz BS, Durand R, Maternal periodontal disease and spontaneous preterm birthPeriodontology 2000 2007 44:103-12. [Google Scholar]
[5]. Romero R, Espinoza J, Mazor M, The preterm parturition syndrome. In Critchely H, Bennett P, Thornton S (eds)Preterm Birth 2004 LondonRCOG Press:28-60. [Google Scholar]
[6]. Jeffcoat MK, Geurs NC, Reddy MS, Current evidence regarding periodontal disease as a risk factor in preterm birthAnn Periodontol 2001 6:183-88. [Google Scholar]
[7]. Offenbacher S, Boggess KA, Murtha AP, Progressive periodontal disease and risk of very preterm deliveryObstet Gynecol 2006 107:29-36. [Google Scholar]
[8]. Casey ML, Cox SM, Beutler B, Cachectin/tumor necrosis factor-alpha formation in human decidua: Potential role of cytokines in infection induced preterm labourJ Clin Invest 1989 83:430-6. [Google Scholar]
[9]. Romero R, Durum SK, Dinarello CA, et al. Interleukin-1: A signal for the initiation of labour in chorioamnionitis. 33rd Annual Meeting for the Society for Gynecologic Investigation, Toronto, Ontario, 1986 [Google Scholar]
[10]. Van Winkelhoff AJ, Loos BG, Van der Reijden WA, Porphyromonas gingivalis, Bacteroides forsythus and other putative periodontal pathogens in subjects with and without periodontal destructionJ Clin Periodontol 2002 29(11):1023-28. [Google Scholar]
[11]. Madianos PN, Lieff S, Murtha AP, Maternal periodontitis and prematurity, II: maternal infection and fetal exposureAnn Periodontol 2001 6(1):175-82. [Google Scholar]
[12]. Offenbacher S, Jared HL, O’Reilly PG, Potential pathogenic mechanisms of periodontitis associated pregnancy complicationsAnn Periodontol 1998 3(1):233-50. [Google Scholar]
[13]. Anderson KV, Jurgens G, Nusslein-Volhard C, Establishment of dorsal-ventral polarity in the Drosophila embryo: genetic studies on the role of the Toll gene productCell 1985 42:779-89. [Google Scholar]
[14]. Dunne A, O’Neill LAJ, The interleukin-1receptor/Toll-like receptor superfamily: signal transduction during inflammation and host defenseSci STKE 2003 171:re3 [Google Scholar]
[15]. Takeda K, Akira S, Toll-like receptors in innate immunityInt Immunology 2005 17(1):1-14. [Google Scholar]
[16]. Akira S, Takeda K, Toll-like receptor signallingNat Rev Immunol 2004 4:499-509. [Google Scholar]
[17]. Kusumoto Y, Hirano H, Saitoh K, Human gingival epithelial cells produce chaemotactic factors interleukin-8 and monocyte chaemoattractant protein-1 after stimulation with Porphyromonas gingivalis via toll-like receptor 2J Periodontol 2004 75:370-79. [Google Scholar]
[18]. Uehara A, Sugawara S, Tahada H, Priming of human oral epithelial cells by interferon gamma to secrete cytokines in response to lipopolysaccharides, lipoteichoic acids and peptidolycansJ Med Microbiol 2002 51:626-34. [Google Scholar]
[19]. Kinane DF, Shiba H, Stathopoulou PG, Gingival epithelial cells heterozygous for Toll-like receptor 4 polymorphisms Asp299Gly and Thr399ile are hypo-responsive to Porphyromonas gingivalisGenes Immun 2006 7(3):190-200. [Google Scholar]
[20]. Sarah SM, Tamilselvan S, Kamatchiammal S, Expression of Toll-like receptors 2 and 4 in gingivitis and chronic periodontitisIndian J Dent Res 2006 17(3):114-16. [Google Scholar]
[21]. Tabeta K, Yamazaki K, Akashi S, Toll-like receptors confer responsiveness to lipopolysaccharide from Porphyromonas gingivalis in human gingival fibroblastsInfect Immun 2000 68:3731-35. [Google Scholar]
[22]. Holmlund U, Cebers G, Dahlfors AR, Expression and regulation of the pattern recognition receptors Toll-like receptor-2 and Toll-like receptor-4 in the human placentaImmunology 2002 107:145-51. [Google Scholar]
[23]. Ma Y, Krikun G, Abrahams VM, Cell type-specific expression and function of Toll-like receptors 2 and 4 in human placenta: implications in fetal infectionPlacenta 2007 28(10):1024-31. [Google Scholar]
[24]. Riley JK, Nelson DM, Toll-like receptors in pregnancy disorders and placental dysfunctionClinical Reviews in Allergy and Immunology 2009 :1-9. [Google Scholar]
[25]. Uh A, Nicholson RC, Gonzalez GV, Lipopolysaccharide stimulation of trophoblasts induces corticotrophin releasing hormone expression through MyD88Am J Obst Gyn 2008 199(30):317 [Google Scholar]
[26]. Shimizu T, Kida Y, Kuwano K, Ureaplasma parvum lipoproteins, including MB antigen, activate NF-κB through TLR1, TLR2 and TLR6Microbiology 2008 154(5):1318-25. [Google Scholar]
[27]. Abrahams VM, Potter JA, Bhat G, Bacterial modulation of human fetal membrane Toll-like receptor expressionAm J Reprod Immunol 2013 69(1):33-40. [Google Scholar]
[28]. Arce RM, Barros SP, Wacker B, Increased TLR4 expression in murine placentas after oral infection with periodontal pathogensPlacenta 2009 30(2):156-62. [Google Scholar]
[29]. Chaparro A, Blanlot C, Ramırez V, Porphyromonas gingivalis, Treponema denticola and toll-like receptor 2 are associated with hypertensive disorders in placental tissue: a case–control studyJ Periodont Res 2013 48:802-09. [Google Scholar]
[30]. Erridge C, Stewart J, Poxton IR, Monocytes heterozygous for the Asp299Gly and Thr399Ile mutations in the Toll-like receptor 4 gene show no deficit in lipopolysaccharide signallingJ Exp Med 2003 197:1787-91. [Google Scholar]
[31]. Arbour NC, Lorenz E, Schutte BC, TLR4 mutations are associated with endotoxin hyporesponsiveness in humansNat Genet 2000 25:187-91. [Google Scholar]
[32]. Varner MW, Esplin MS, Current understanding of genetic factors in preterm birthBJOG 2005 112(suppl 1):28-31. [Google Scholar]
[33]. Adams Waldorf KM, Persing D, Novy MJ, Pre-treatment with Toll-like receptor 4 antagonist inhibits lipopolysaccharide-induced preterm uterine contractility, cytokines, and prostaglandins in rhesus monkeysReprod Sci 2008 15(2):121-27. [Google Scholar]
[34]. Xiong X, Buekens P, Goldenberg RL, Optimal timing of periodontal disease treatment for prevention of adverse pregnancy outcomes: before or during pregnancy?Am J Obstet Gynecol 2011 205:e1-6. [Google Scholar]
[35]. Polyzos NP, Polyzos IP, Zavos A, Obstetric outcomes after treatment of periodontal disease during pregnancy: systematic review and meta-analysisBMJ 2010 29:341 [Google Scholar]
[36]. Michalowicz BS, Gustafsson A, Thumbigere-Math V, The effects of periodontal treatment on pregnancy outcomesJ Periodontol 2013 84(4):S195-208. [Google Scholar]