Among chemical plaque control agents, Chlorhexidine digluconate is considered as a gold standard against which other chemical plaque control agents can be compared to gauge their efficacy [6]. Chlorhexidine (CHX), a dicationic chlorophenyl biguanide is an antiseptic agent effective against various anaerobic and aerobic bacteria including Streptococcus mutants, Streptococcus aureus, Porphyromans gingivalis and Prevotella intermedia [7,8]. The primary mechanism of action of CHX involves membrane disruption, causing concentration-dependent growth inhibition and bacterial cell death. The cationic nature of CHX enables it to bind to tooth surfaces and oral mucosa, reducing pellicle formation and resulting it to elicit is effect over a period of time referred to as substantivity thereby reducing bacterial viability and inhibiting plaque growth [9]. There are however associated side effects due to its prolonged use such as extrinsic staining of the teeth, dysgeusia, oral mucositis and salivary gland atresia [7].
The present study was conducted as a single center randomized triple blinded prospective cross over study in a dental cohort to assess the efficacy of commercially available ClO2 mouth rinse in inhibiting plaque regrowth as compared to CHX using a four day plaque regrowth model [11]. The study also assessed the effect on tongue coat accumulation through Winkel’s tongue coating index and wet tongue coat weight. Oral microbial load was also evaluated through the number of Colony Forming Units (CFUs) from plaque samples collected from teeth surfaces and buccal mucosa.
Materials and Methods
Study Population
Ethical clearance was obtained from the Institutional Ethical Committee. The sample size of the study was decided on the basis of previous reference articles [14,15] and number of respondents ready for the participation. The study was conducted within the institution and restricted to third year dental students whose population was 100; 20% of the population was considered by the statistician for primary analysis.
After being explained about the purpose, products to be used in study with possible side effects and study design [Table/Fig-1], 25 healthy dental student volunteers (11 males, 14 females) aged between 18-25 years (mean age = 19.8 years) consenting to their participation in the study by signed informed consent forms were enrolled for the study once they met the inclusion criteria.
Flow chart for the study events
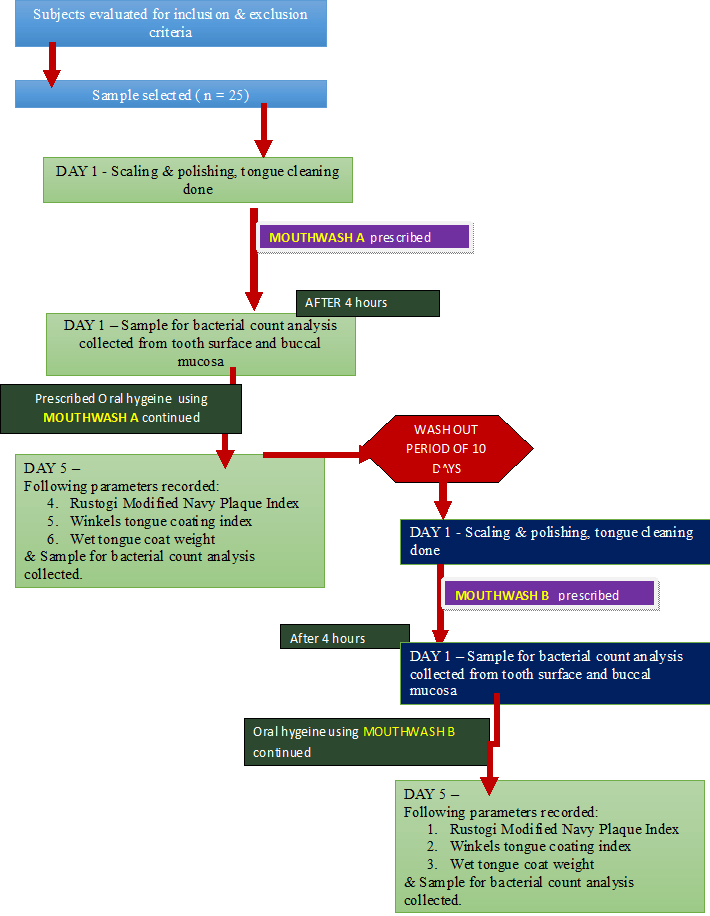
The subject’s selection criteria included, systemically healthy individuals, presenting good level of oral hygiene evaluated on the basis of a mean plaque index (Pl) score < 1 (Sillness & Loe 1967) [16] with presence of minimum 20 natural scorable teeth of permanent dentition excluding third molars and subjects who were willing to abstain from any other means of oral hygiene maintenance throughout the investigation period.
The exclusion criteria of the study included subjects that presented with signs of gingival or periodontal disease, gave history of any form of non-surgical and /or surgical periodontal therapy within the past 6 months, had taken antimicrobials and anti-inflammatory drug therapy within the past one month, had given history of being under oral contraceptives or had history of drug dependence and substance abuse. Subjects having unrestored teeth, orthodontic and prosthetic devices and habits like mouth breathing, bruxism and clenching were excluded from the study. Subjects with physical debilitation were also excluded from the study.
The following were compared
Mouthrinse A - Commercially available stabilized ClO2 mouthrinse in aqueous vehicle Freshchlor® (Rowpar group pharmaceuticals, Bangalore, India.)
Mouthrinse B – Commercially available 0.2% CHX gluconate mouthrinse in aqueous vehicle Hexidine® (ICPA, Bangalore, India).
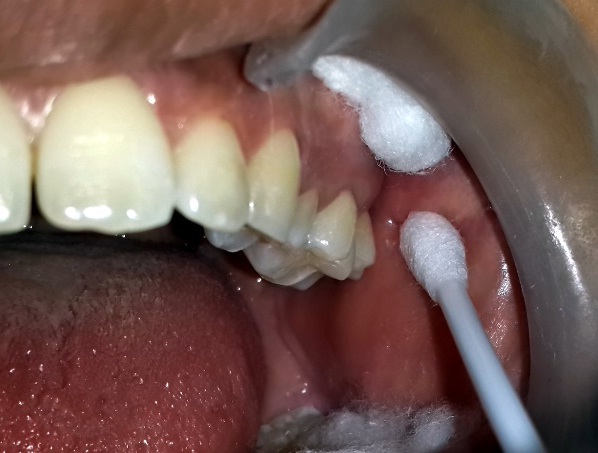
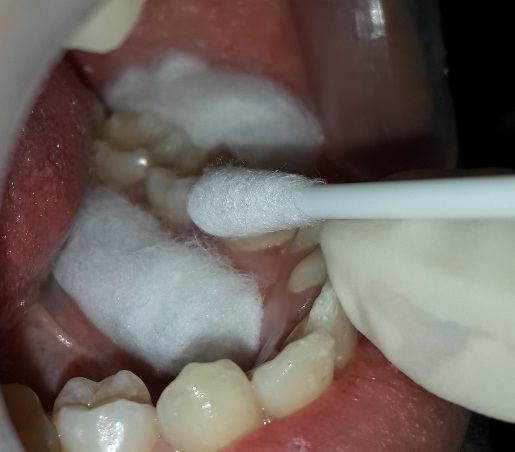
For the study to be blinded, the mouthrinses were supplied in identical white opaque bottles along with measuring caps labelled as mouthrinse A and mouthrinse B. At baseline of both the test phases, all participants received a thorough scaling and polishing by ultrasonic scalers and tongue coat removal using toothbrushes on the dorsum of the tongue until the examiner visually confirmed that all the tongue coating was completely removed. Following which the subjects were asked to rinse and gargle for one minute with the allocated mouthrinse under supervision. Four hours after the first rinse the smears were taken from the buccal mucosa [Table/Fig-2] and from the tooth surface i.e. the lingual surface of lower left first molar [Table/Fig-3].
Collection of sample using cotton swab from buccal mucosa
Collection of plaque sample using cotton swab from tooth surface
During each day of the ensuing four day period, subjects were instructed verbally along with written instructions to suspend all routine oral hygiene procedures and to rinse and gargle twice daily in morning and evening for one minute with 10 ml of undiluted solution of their allocated mouthrinse using a stopwatch. Subjects were also asked to document their rinsing records and report any unexpected occurrence in the dairy provided. The last rinse was performed in the evening of day four. Compliance with the prescribed regime was assessed by means of the patients rinsing record and by measuring the residual mouth rinse in the returned mouthrinse bottles on the fifth day.
In the clinical evaluation carried out on the fifth day from the baseline, the plaque sample from the tooth surface and buccal mucosa were collected, following which the tongue coat score was recorded using Winkel’s tongue coating index [17] [Table/Fig-4].
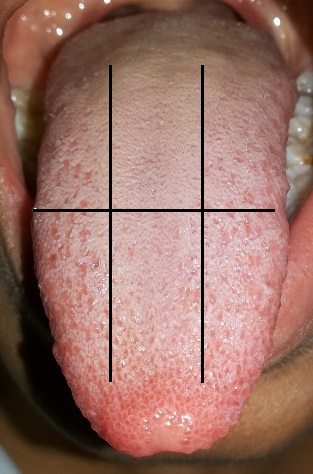
Winkel’s tongue coat index scoring by dividing the tongue into 6 notional segments
0 – No coating
1 – Thin coating
2- Thick coating
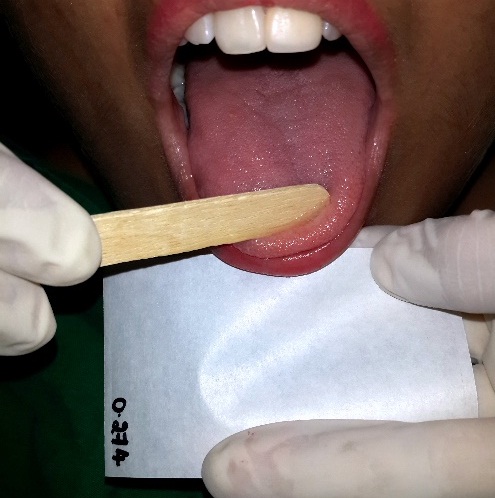
For measuring the wet tongue coat weight, the tongue coat was collected on a 2 inch x 2 inch pre-weight moisture impervious paper sheet with the help of ice cream stick [18] [Table/Fig-5]. The full mouth plaque index was then recorded using Rustogi et al., Modified Navy Plaque Index [19] for which the subjects were asked to swish for one minute with 2% erythrosine disclosing agent. After the assessment, scaling and polishing and tongue coat removal was performed for each volunteer. A total of two experimental phases in the cross over design were conducted with an intervening wash out period of ten days [20], during which the subjects resumed their routine oral hygiene maintenance.
Collection of tongue coat using ice-cream stick on a moisture impervious paper sheet
Measurement Parameters
Clinical measurements
Extent of tongue coat: This was scored using Winkel’s tongue coating index [17]. The subjects were asked to protrude their tongue as far as possible. Saliva on the dorsum of the tongue was removed by blotting the surface with the absorbent paper. The tongue dorsum was notionally divided into six sections so as to obtain one middle and two lateral areas for anterior as well as posterior of tongue. Each section was scored for the presence of tongue coat if only the tongue coat was covering more than 1/3 of each section. No coating present was given score 0. A light-thin coating was given score 1 (the pink color underneath the coating is still visible). Heavy-thick coating was given score 2 (no pink color can be observed under the coating). The tongue coating value was obtained by adding all six scores, obtaining a total score in 0 to 12 range.
Wet weight of tongue coat [18]: Following removal of the saliva by blotting the tongue dorsum with absorbent paper as described above, a sterile ice cream stick was used to scrape and collect the tongue coatings on 2 inch x 2 inch pre-weighed moisture impervious paper sheet. Repeated back, forth and sideway scraping movements in different directions were continued until no more coatings could be dislodged. The wet weight of the collected tongue coating was measured to the nearest 0.01g by subtracting the pre-scraping weight of the moisture impervious paper.
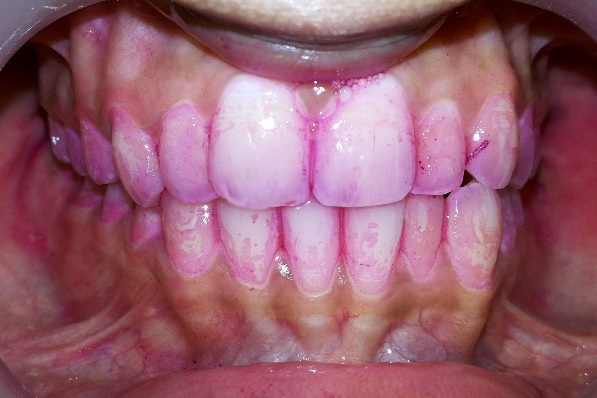
Rustogi Modified Navy Plaque Index [19]: The buccal and lingual tooth surface of all teeth except the third molars was divided into nine areas. Disclosed plaque [Table/Fig-6] was scored in each tooth area as present (scored as 1) or absent (scored as 0). The full mouth score was given by addition of all surface scores divided by surfaces examined.
Disclosed plaque for scoring according to Rustogi Modified Navy Plaque Index
Microbial analysis: The antibacterial effects of the mouthrinses were assessed microbiologically by measuring the Colony Forming Units (CFUs) per sample collected from the tooth surface and from the buccal mucosa. The samples were collected at baseline, four hours after the first rinse and on day five from baseline. To take the sample from the tooth surface, the quadrant was first isolated with cotton wool rolls and the lingual surface of the teeth was dried with a gentle stream of air. A cotton wool swab (Micromaster Laboratories Pvt Ltd, Mumbai, India) was stroked across the entire lingual tooth surface from mesial to distal while exerting pressure on the swab; the attempt was made to get as close as possible to the gingival margin without touching it. Sample from the mucosa were taken from the buccal mucosa opposite to upper left first molar and above the secretory duct of the parotid gland using cotton wool swabs after isolating the upper and lower buccal vestibule with the cotton rolls. The collected swabs were stored in 5-ml Tryptone Soya Broth [Table/Fig-7] (Micromaster Laboratories Pvt Ltd, Mumbai, India) in a refrigerator until analysis, which was done in the afternoon of the same day. For the analysis, the samples were vortex mixed for 30s, and 100 μl of the 10-2 dilutions (Phosphate buffered saline) were used to inoculate Tryptone Soya Broth agar plates (Micromaster Laboratories Pvt Ltd, Mumbai, India) using spread plate method. The plates were then incubated at 370C for 48h. Results were expressed as colony forming units per sample (CFU/sample) recorded with the help of a digital colony counting pen.
Cotton swab samples stored in 5ml of Tyrptone Soya broth

Statistical Analysis
The Data was analysed using SPSS 16.00 and presented using descriptive statistics. Independent t-test was used for the intergroup comparison between the two groups. The level of significance used for the analysis was 5%. The p-value less than that of 0.05 was treated as significant.
Results
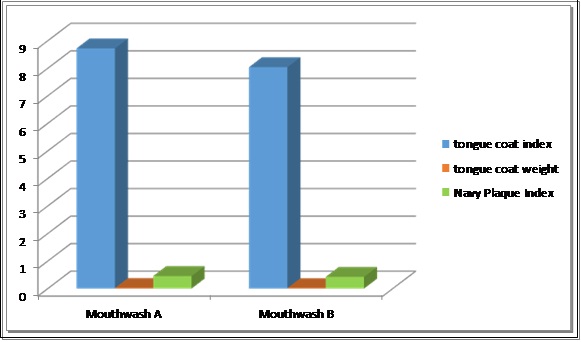
All subjects completed the four day experimental plaque regrowth model period. The mean plaque scores, CFU at four hours and four days from mucosa and plaque sample, tongue coat weight, tongue coat index for both the mouthrinse at the end of experimental period are displayed in [Table/Fig-8].
Mean scores and p value for the parameters assessed
| Parameters assessed | Test Groups | n | Mean | Std. Deviation | Std. Error Mean | p -value |
|---|
| Rustogi Modification of Navy Plaque Index | Mouthwash A | 25 | 0.4548 | 0.07187 | 0.01437 | 0.050 |
| Mouthwash B | 25 | 0.4250 | 0.01855 | 0.00371 |
| CFU obtained from mucosal sample four hours after the first rinse | Mouthrinse A | 25 | 45.2800 | 7.05644 | 1.41129 | • 0.00(6.244E-5) |
| Mouthrinse B | 25 | 37.6400 | 5.09804 | 1.01961 |
| CFU obtained from plaque sample four hours after the first rinse | Mouthrinse A | 25 | 35.8800 | 5.16656 | 1.03331 | 0.001 |
| Mouthrinse B | 25 | 30.6800 | 4.82804 | .96561 |
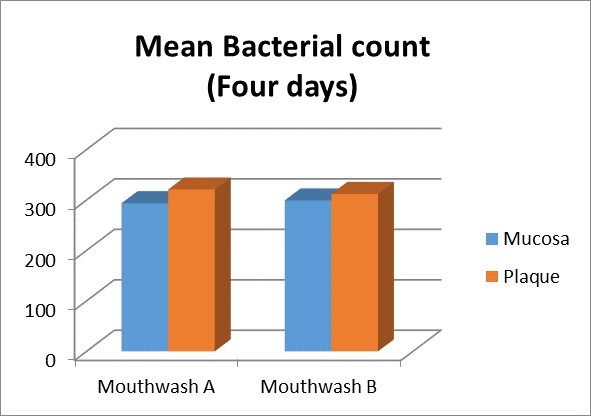
| CFU obtained from plaque sample after four days | Mouthwash A | 25 | 320.6800 | 19.90335 | 3.98067 | 0.160 |
| Mouthwash B | 25 | 312.1200 | 22.47280 | 4.49456 |
| CFU obtained from plaque sample after four days | Mouthwash A | 25 | 299.6800 | 21.65010 | 4.33002 | -5.56000 |
| Mouthwash B | 25 | 290.2400 | 21.32346 | 4.26469 |
| Tongue coat weight | Mouthwash A | 25 | .0180 | .00707 | .00141 | 0.238 |
| Mouthwash B | 25 | .0156 | .00712 | .00142 |
| Winkel’s tongue coating ndex | Mouthwash A | 25 | 8.7200 | 1.10000 | .22000 | 0.065 |
| Mouthwash B | 25 | 8.0400 | 1.42829 | .28566 |
Intragroup comparison of post experimental period plaque scores by Rustogi Modified Navy Plaque index, tongue coat weight and Winkels tongue coat Index revealed no statistically significant difference between these parameters on usage of mouthrinse A and mouthrinse B [Table/Fig-9].
Mean scores of Tongue coat index, Tongue coat weight, Rastogi modification of Navy Plaque Index obtained after the four day experimental
The CFU from the mucosa and plaque [Table/Fig-10a,b] samples collected after four hours from the first rinse was significantly lower for both the mouthrinse when compared with the CFU obtained after four days. However on intergroup comparison for tooth plaque and mucosal sample collected after four hours Mouthrinse B showed lesser CFU than Mouthrinse A [Table/Fig-8,11] which was statistically significant.
CFU obtained from plaque sample four hours after rinsing with mouthrinse A
CFU obtained from plaque sample four hours after rinsing with mouthrinse B
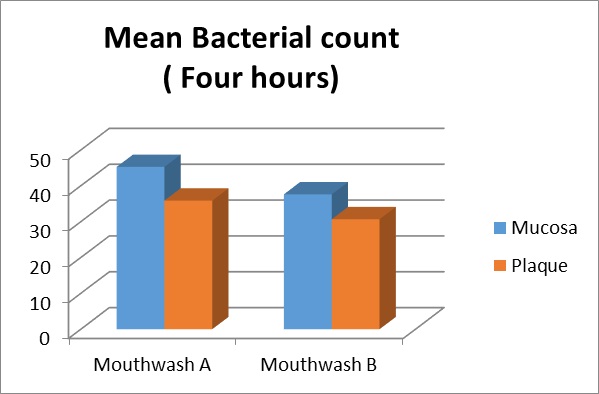
Mean bacterial count four hours after the first rinse with the study test products
Intergroup comparison for CFU between Mouthrinse A and Mouthrinse B obtained from tooth plaque [Table/Fig-12a,b] and buccal mucosa. [Table/Fig-8,13] sample collected after four days revealed no statistically significant difference indicating that average CFU from four days samples were almost equal in both the mouthrinses.
CFU obtained from plaque sample after 4 days of rinsing with mouthrinse A
CFU obtained from plaque sample after 4 days of rinsing with mouthrinse B
Mean bacterial count four days after the first rinse with the study test products
Discussion
The present study was designed to compare the plaque inhibitory effect of commercially available stabilized CIO2 and 0.2% chlorhexidine in a four day plaque regrowth model by evaluation of the scores of Rustogi Modification of Navy Plaque Index. Assessment of amount of tongue coat accumulation was based on scores of Winkels tongue coating index and wet tongue coat weight and comparison of the antibacterial property of the two formulations in reducing the oral bacterial load was made with the help of viable colony forming unit per sample collected from the tooth and buccal mucosa.
The four-day experimental period length was chosen because measurable volume of plaque accumulation is reached after four to five days of no oral hygiene [21]. Other advantages offered by adaptation of this model involves removal of the confounding variables, such as the Hawthorne effect and influence of pre-study prophylaxis [22,23]. This model has been used in various studies to assess the efficacy of many mouthwashes [11,15,24].
In the oral cavity apart from teeth, the oral mucosa and the dorsum of the tongue accounts for about 80% of the oral surfaces which allow bacterial colonization by formation of plaque biofilm which act as a potent reservoir for pathogenic bacteria, which can re–colonize the teeth; thus facilitating development of periodontal disease [25]. Faveri et al., studied the microbiota of the dorsum of tongue in dental students and suggested that the tongue surface could be an important reservoir for periodontal pathogens and may play a role in the recolonization of tooth surfaces [26]. Gross et al., suggested reduction in plaque accumulation on tooth surface upon tongue cleaning [27]. These findings suggest a relationship between tongue coat formation and dental plaque formation. Therefore the effect of the two compared mouthrinses on the extent of tongue coat formation by Winkels tongue coat index which registers the tongue coating on a quantitative scale and by tongue coat wet weight was evaluated. To rule out the carry over effect of the mouthrinses a wash out period of ten days was given between the two experimental phases. CIO2 being highly soluble in water is usually and preferably dispensed in an aqueous base; so, we also chose 0.2% CHX mouthrinse in aqueous base thus reducing the confounding factor that alcohol containing mouthrinse have superior antibacterial property over aqueous base as suggested by previous studies [28,29]. The oral bacterial load was measured in the study with the help of samples collected from mucosa and tooth at two points of time which also gives some information on the relative substantivity of the two test formulations. In this study samples were collected from representative areas where plaque growth was more likely to occur [30,31].
The samples collected after four hours from tooth surface measured early colonization of bacteria and/or salivary contamination and the CFU count from it is indicative of persistence of antibacterial activity of the two tested mouthrinses. The sample tested on the fifth day was indicative of the amount of bacteria that were neither dead nor inactivated about 12 h after the last rinse. The results obtained revealed that the number of colony forming unit were significantly more in Mouthrinse A containing stabilized CIO2 group when compared to mouthrinse B group containing 0.2% CHX after four hours, however on comparison of CFU obtained from four days plaque and mucosal samples indicated that though the CFU were more with Mouthrinse A, however the obtained value for the two test formulations did not show a statistically significant difference. These findings obtained in our study are in accordance to the previous study carried out by Goultschin et al., suggesting that ClO2 has low substantivity as opposed to chlorhexidine [32].
Available literature on effect of ClO2 mouthrinse on plaque accumulation and its bacterial viability is limited. Spiros et al., conducted a study to assess the plaque growth inhibition of a ClO2-containing mouthrinse compared to a CHX-containing mouthrinse during a three day de novo plaque-accumulation model [33]. The results of the study revealed that CHX inhibited plaque growth significantly more than the mouthrinse containing CIO2. The results of our study are not in agreement with these findings. However the results of the study by Spiros et al., also indicate a preference towards usage of ClO2-containing mouthrinse attributed to its taste and less taste alterations experienced by the subjects. Another doubled masked, cross-over study carried out by Yates et al., to evaluate and compare the persistence of antimicrobial action and plaque inhibitory properties of three Acidified sodium chlorite (ASC) mouthrinses, when compared with positive control, CHX and placebo control, water, rinses inferred that the 3 ASC rinses have equivalent plaque inhibitory action to chlorhexidine as a mouthrinse [14]. These findings are in accordance to the results obtained in our study which state that though the plaque inhibitory effect and reduction of CFU was more with Mouthrinse B, the intergroup comparison did not reach a statistically significant difference suggesting that the plaque inhibitory property, rate of tongue coat accumulation and reduction in oral microbial load was almost equal for the two compared mouthrinses.
Limitations of The Study
The four-day plaque regrowth model used in the study imparts information of only on the early colonizers of plaque.
The study employs a quantitative assay to determine the effect of the tested mouthrinses on oral bacteria load, however application of qualitative assay to determine the effect on oral bacterial load can further render more information on the effect of the tested mouthrinse on the microbiota present in the dental plaque and buccal mucosa.
Conclusion
Our study concludes that the plaque inhibitory property, rate of tongue coat accumulation, antibacterial property of chlorine dioxide mouthrinse is comparable to the 0.2% Chlorhexidine gluconate mouthrinse. However, further studies of longer duration, large sample size, with other comparable products (negative and positive controls) and involving qualitative analysis of oral bacterial samples are recommended to establish the effectiveness of stabilized chlorine dioxide mouthrinse among the other available chemical plaque control agents.