Serum Adenosine Deaminase as Inflammatory Marker in Rheumatoid Arthritis
Kiranmayi S. Vinapamula1, Srinivasarao V.L.N. Pemmaraju2, Siddartha Kumar Bhattaram3, Aparna R. Bitla4, Suchitra M. Manohar5
1 Assistant Professor, Department of Biochemistry, Sri Venkateswara Institute of Medical Sciences, Tirupati, Andhra Pradesh, India.
2 Professor and Head, Department of Biochemistry, Sri Venkateswara Institute of Medical Sciences, Tirupati, Andhra Pradesh, India.
3 Professor, Department of Medicine, Sri Venkateswara Institute of Medical Sciences, Tirupati, Andhra Pradesh, India.
4 Associate Professor, Department of Biochemistry, Sri Venkateswara Institute of Medical Sciences, Tirupati, Andhra Pradesh, India.
5 Associate Professor, Department of Biochemistry, Sri Venkateswara Institute of Medical Sciences, Tirupati, Andhra Pradesh, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Srinivasarao V.L.N. Pemmaraju, Professor and Head, Department of Biochemistry, Sri Venkateswara Institute of Medical Sciences, Tirupati, AP-517507, India.
E-mail: seenupvln@yahoo.com
Background
Rheumatoid arthritis (RA) is a prototypical inflammatory joint disease. The degree of inflammation is reflected in the extent of joint damage, which further has influence on the quality of life of patients with RA, including risk of atherosclerosis. Hence, besides clinical indices, estimation of degree of inflammation using biochemical markers helps in effecting optimum treatment strategies. C-reactive protein (CRP) is established as an inflammatory marker in patients with RA. Adenosine deaminase (ADA), an enzyme of purine metabolism is considered as a marker of cell mediated immunity and has also been suggested as a marker of inflammatory process in RA. The present study attempts to study the efficacy of serum ADA activity as an inflammatory marker in RA.
Materials and Methods
Forty six RA patients and forty six age and sex matched healthy controls were included in the study. ADA activity and high sensitivity C-reactive protein (hsCRP) levels in serum were measured in all the subjects. Statistical analyses were done using Medcalc statistical software version 12.2.2.
Results
ADA activity and hsCRP levels were increased in RA patients compared to controls (p<0.0001 and 0.0001 respectively). Significant positive correlation was obtained between hsCRP and ADA in patients (r=0.316, p=0.033). Receiver operating characteristic (ROC) curve analysis revealed statistically significant area under curve (AUC) for ADA that is comparable to that obtained for hsCRP (0.776, p<0.0001 for ADA, 0.726, p<0.0001 for hsCRP). Similar diagnostic utility was obtained with ROC generated cut-off value of 25.3 IU/L (82.6% sensitivity and 65.2% specificity) and with control mean value of 23.48 IU/L (86.96% sensitivity and 54.35% specificity) for ADA.
Conclusion
Findings of the present study indicate the importance of ADA as a marker of inflammation. Considering the higher sensitivity obtained, we propose control mean (23.48 IU/L) as a cut-off for serum ADA activity as an inflammatory marker. Owing to the simplicity and also the cost effectiveness of ADA assay, ADA may be recommended as a marker of inflammation in patients with RA. However, further larger and well controlled studies are needed to establish its role as inflammatory marker.
ADA, Autoimmune disease, Inflammatory joint disease, Inflammation
Introduction
Rheumatoid arthritis (RA) is a chronic systemic inflammatory disease, primarily affecting the joints of hands and feet causing erosive, symmetrical polyarthritis. Although the exact aetiology of RA is unknown, it is considered to be an autoimmune disease [1]. Various measures are used for the evaluation of disease activity in rheumatoid arthritis and laboratory tests such as ESR and CRP are being used as markers of inflammation [2]. CRP is an acute phase protein synthesized by hepatocytes in response to stimulation by proinflammatory cytokines, particularly IL-6 [3]. Although a general marker of systemic inflammation, CRP has been established as a promising inflammatory marker in RA [4] and measurement of serum CRP concentration as a measure of disease activity is being practiced since a long time. High sensitivity CRP (hsCRP) testing has been recommended to measure low disease activity in RA which is associated with poor long term outcome, since routine CRP testing may not detect the low grade systemic inflammation that is commonly observed in RA [4]. Although a prototypical inflammatory disease, both immune and inflammatory processes play an important role in the pathogenesis of RA [5]. Purine metabolism may be related to rheumatoid arthritis since several disturbances of purine metabolism have been found to be associated with immune disorders [6]. Immune dysregulation forms one of the pathogenic mechanisms of rheumatoid arthritis and hence, it is not surprising to find altered levels of purine enzymes in RA. Adenosine deaminase (ADA, adenosine amino hydrolase E.C. 3.5.4.4) is an important enzyme of purine metabolism catalysing the irreversible deamination of adenosine to form inosine [7] and is considered as a marker of cell mediated immunity [5]. Serum ADA was found to reflect monocyte/macrophage activity in inflammatory conditions such as RA and has also been suggested as a marker of inflammatory processes in RA [8]. It is well-known that continued disease activity results in joint damage, decreased physical activity or even irreversible disability [9]. Hence, early diagnosis and intervention help in reducing the morbidity associated with RA. Besides clinical indices such as Disease activity score (DAS), evaluation of inflammatory biomarkers provides a means of objectively estimating the degree of inflammation, thereby allowing optimised treatment plans. In this background, the present study attempts to study the efficacy of ADA as an inflammatory marker in patients with rheumatoid arthritis.
Materials and Methods
Forty six patients diagnosed with Rheumatoid arthritis as per 1987 revised American Rheumatology Association criteria [10] and forty six age and sex matched healthy controls were included in the study after informed consent. The study was conducted between January and July 2011. The study was approved by institutional ethics committee. Blood samples were collected from all the subjects after an overnight fast, serum separated and stored at -80oC until analysis. Serum ADA activity (Tulip diagnostics (P) Ltd, India) was measured using colorimetric method of Guisti and Galanti [11]. hsCRP (APTEC diagnostics nv, Belgium) was assayed by immunoturbidimetry. Analyses were done on Perkin Elmer Lambda 1.2 UV Vis double beam spectrophotometer (ADA) and Beckman Synchron CX5 fully automated analyser, USA (hs CRP).
Statistical Analysis
Continuous data were expressed as mean ± SD and median (inter quartile range) for normally distributed and skewed data respectively. Mann Whitney U-test was used to study the differences between controls and patients for the parameters studied. Association between ADA and hsCRP was analysed using Spearman rank correlation. Receiver operating characteristic (ROC) curves were constructed to study the diagnostic utility of ADA as inflammatory marker. A p<0.05 was considered statistically significant. Analysis was performed using Medcalc statistical software version 12.2.2.
Results
Serum ADA and hsCRP levels were found to be significantly higher in RA patients compared to controls [Table/Fig-1]. Spearman rank correlation analysis showed significant positive correlation between ADA and hsCRP (r=0.316, p=0.033). When the diagnostic utility of ADA as an inflammatory marker was assessed using ROC curve analysis, ADA showed significant AUC (0.776, p<0.0001), comparable to that of hsCRP (0.726, p<0.0001;[Table/Fig-2,3]). When a comparison of diagnostic utility of ADA and hsCRP was performed using ROC curve analysis, ADA showed a slightly higher but statistically insignificant AUC (0.050, p=0.458; [Table/Fig-2]). A cut-off value of 25.3 IU/L was obtained for ADA with the best combination of 82.6% sensitivity and 65.2% specificity; for hsCRP, a cut-off value of 1.14 mg/L was obtained at the best level of 45.7% sensitivity and 93.5% specificity. However, when we calculated the sensitivity and specificity of ADA as an inflammatory marker using control mean (23.48 IU/L) as cut-off, we obtained a sensitivity and specificity of 86.96% and 54.35% respectively [Table/Fig-4].
Demographic characteristics, ADA and hsCRP levels in controls and RA patients.
| parameter | ControlsN=46 | RA patientsN=46 | p-value |
|---|
| Age (years) | 40.15±11.59 | 42.33±12.62 | 0.259 |
| BMI (kg/m2) | 20.25±1.28 | 20.70±2.66 | 0.117 |
| M/F | 5/41 | 5/41 | – |
| ADA (U/L) | 23.48±7.59 | 32.79±10.13 | <0.0001** |
| hsCRP (mg/L)* | 0.21(0.08-0.59) | 0.94(0.21-1.95) | 0.0002** |
Data expressed as mean ± SD, * median (inter quartile range), ** statistical significance
RA-rheumatoid arthritis; BMI- body mass index; M/F- male/female; ADA- adenosine deaminase; hsCRP- high sensitivity C- reactive protein
ROC curve analysis of ADA, hsCRP and comparison of ADA and hsCRP.
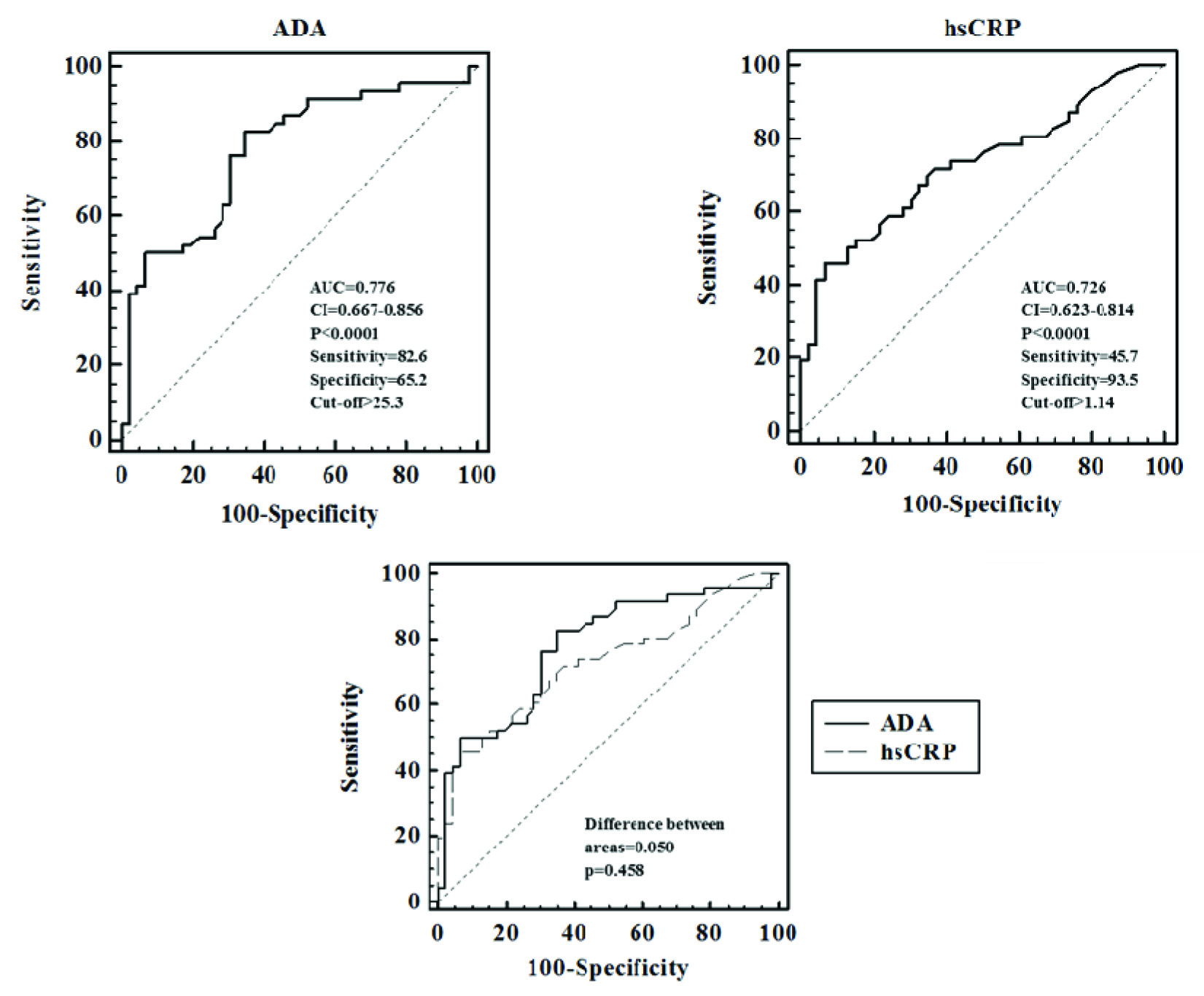
ROC curve analysis of ADA and hsCRP levels.
| Parameter | AUC | p-value | 95% CI |
|---|
| Lower bound | upper bound |
|---|
| ADA | 0.776 | <0.0001 | 0.667 | 0.856 |
| hsCRP | 0.726 | <0.0001 | 0.623 | 0.814 |
ADA- adenosine deaminase; hsCRP- high sensitivity C- reactive protein
Sensitivity and specificity values for ADA using various cut-off values
| Parameter | ROC cut-off(25.30 IU/L) | Control mean(23.48 IU/L) |
|---|
| Sensitivity (%) | 82.61 | 86.96 |
| Specificity (%) | 65.20 | 54.35 |
Discussion
Since prevention of joint damage and decreasing the morbidity form important components of management of RA patients, early initiation of appropriate treatment is required. Although clinical indices help in assessing disease activity, biochemical markers also act as indicators of disease activity and progression. CRP measurement has been suggested to be beneficial for estimating short term changes in disease progression in RA patients [12,13]. Several studies have reported increased CRP levels in patients with RA [4,14]. In the present study serum levels of both ADA and hsCRP were significantly elevated in RA patients compared to controls. Similar findings were reported earlier [5]. Although the exact cause of increase in ADA levels is not known, the activity may be increased due to its release from damaged cells and increased cellular proliferation in RA [15]. Synthesis of CRP in liver is triggered by pro inflammatory cytokines released from monocyte and/or macrophages. The pro inflammatory response leads to secretion of Interleukin-1β and Tumour necrosis factor-α which further results in the release of Interleukin-6, a messenger cytokine which stimulates liver to secrete CRP [5].
When the association between ADA and hsCRP was analysed in the present study, serum ADA showed significant positive correlation with hsCRP in RA patients. Studies conducted to evaluate the role of ADA in patients with rheumatoid arthritis showed varied results. Nalesnik M et al., reported significant correlation between ADA and CRP in patients with RA without methotrexate treatment and suggested that ADA activity in serum can be used as a biochemical marker of the inflammatory process in RA [16]. In an earlier study, Yuksel and colleague [17] measured serum and synovial fluid ADA activity in patients with Rheumatoid arthritis, osteoarthritis and reactive arthritis. They did not observe significant difference among the study groups with respect to serum ADA activity. However, they found significant association between serum and synovial ADA activity in RA patients and concluded that measurement of synovial fluid ADA activity may be helpful in differentiating between RA, osteoarthritis and reactive arthritis in certain conditions. A study by Sari et al., [18] showed that serum total ADA activity is associated with RA and may provide a useful adjunct to assess inflammation besides traditional indices. Similarly, Surekha Rani et al., [5] also reported that measurement of ADA besides CRP levels helps in the better management RA patients. On contrary, Demir G et al., [8] reported that although ADA levels were higher in their RA patients than in controls, ADA was not related to any of the disease activity parameters and hence concluded that traditional parameters and not ADA activity form the reliable markers to determine the disease activity in RA. On the other hand, recently, Haque SS and colleagues [19] who found significantly increased levels of ADA in RA patients compared to controls and a positive CRP test in a significant number of RA patients in their study suggested that measuring ADA activity helps in a better understanding of some of the pathophysiological aspects of the disease. Thus, although conflicting results were reported with respect to the association of ADA with markers of disease activity, increased ADA levels were observed in RA patients in most of the studies.
ADA catalyzes the irreversible hydrolysis of adenosine to inosine. Adenosine has been shown to be a potent endogenous anti inflammatory agent [20]. The enzyme ADA represents a checkpoint in the regulation of extra cellular Adenosine levels [8] and thus is likely to modulate the inflammatory processes. Hence, although determination of serum adenosine levels is an appropriate way to assess disease activity in RA [8], ADA also seems to be a prediction marker of the inflammatory process in RA [16]. In this context, Zamani et al., [21] studied the relationship between serum adenosine deaminase and disease activity and concluded that a new disease activity index applying serum ADA helps in predicting disease activity in RA.
Minimising inflammation helps to reduce the functional disability and improve the quality of life in patients with RA. In this context, biomarkers help in providing objective measurements of the disease process underlying RA [22]. Studies conducted to determine the utility of ADA as inflammatory marker in rheumatoid arthritis using ROC analysis are limited. Zakeri Z et al., observed a cut-off point of 15 IU/L for serum ADA activity with 93.3% sensitivity and 53.3% specificity and concluded that synovial fluid ADA is a sensitive and specific test for rheumatoid effusions [23]. This is understandable considering that the primary focus of inflammation in RA is synovium. In the present study, ROC curve analysis of ADA as inflammatory marker showed significant AUC (0.776, p<0.0001) with a cut-off value >25.3 IU/L with 82.6% sensitivity and 65.2% specificity, which further support the findings of Zakeri Z et al., However, we obtained a higher cut-off point (25.3 IU/L vs 15 IU/L) which could be due to differences in sample size, degree of inflammation, effect of treatment between the two studies. When we compared the diagnostic utility of ADA with an established inflammatory marker such as hsCRP, we have observed a slightly higher but statistically non-significant AUC for ADA (difference between areas: 0.050, p=0.458; [Table/Fig-2]). Considering the higher sensitivity which helps to identify patients with inflammation better and also considering the cost effectiveness of ADA assay when compared to hsCRP, we propose serum ADA activity (with control mean 23.48 IU/L as a cut-off value, with 86.96% sensitivity and 54.35% specificity) as a useful inflammatory marker in Rheumatoid arthritis.
Conclusion
The findings of the present study signify the role of ADA as a marker of inflammation in RA. The simplicity of measuring ADA activity combined with its cost effectiveness provides an added advantage to consider ADA as an inflammatory marker in patients with rheumatoid arthritis. However, larger well controlled studies are required to further evaluate and establish the role of ADA as an independent marker of inflammation in Rheumatoid arthritis.
Data expressed as mean ± SD, * median (inter quartile range), ** statistical significance
RA-rheumatoid arthritis; BMI- body mass index; M/F- male/female; ADA- adenosine deaminase; hsCRP- high sensitivity C- reactive protein
ADA- adenosine deaminase; hsCRP- high sensitivity C- reactive protein
[1]. Salesi M, Ghazvini RA, Farajzadegan Z, Karimifar M, Karimzadeh H, Masoumi M, Serum adenosine deaminase in patients with rheumatoid arthritis treated with methotrexateJ Res Pharm Pract 2012 1(2):72-76. [Google Scholar]
[2]. Keenan RT, Swearingen CJ, Yazici Y, Erythrocyte sedimentation rate and C-reactive protein levels are poorly correlated with clinical measures of disease activity in rheumatoid arthritis, systemic lupus erythematosus and osteoarthritis patientsClin Exp Rheumatol 2008 26(5):814-19. [Google Scholar]
[3]. Singh HV, Shrivastava AK, Raizada A, Singh SK, Pandey A, Singh N, Atherogenic lipid profile and high sensitive C-reactive protein in patients with rheumatoid arthritisClin Biochem 2013 46(12):1007-12. [Google Scholar]
[4]. Shrivastava AK, Singh HV, Raizada A, Singh SK, Pandey A, Singh N, Inflammatory markers in patients with rheumatoid arthritisAllergol Immunopathol (Madr) 2015 43(1):81-87. [Google Scholar]
[5]. Surekha RH, Madhavi G, Srikhant BMV, Jharna P, Rao URK, Jyothi A, Serum ADA and C-reactive Protein in Rheumatoid ArthritisInt J Hum Genet 2006 6(3):195-98. [Google Scholar]
[6]. van Ede AE, Laan RF, De Abreu RA, Stegeman AB, van de Putte LB, Purine enzymes in patients with rheumatoid arthritis treated with methotrexateAnn Rheum Dis 2002 61(12):1060-64. [Google Scholar]
[7]. Fox IH, Kelley WN, The role of adenosine and 2’-deoxyadenosine in mammalian cellsAnnu Rev Biochem 1978 47:655-86. [Google Scholar]
[8]. Demir G, Borman P, Ayhan F, Ozgün T, Kaygısız F, Yilmez G, Serum Adenosine Deaminase Level is High But Not Related with Disease Activity Parameters in Patients with Rheumatoid ArthritisOpen Rheumatol J 2014 8:24-28. [Google Scholar]
[9]. Aletaha D, Smolen J, Ward MM, Measuring function in rheumatoid arthritis: Identifying reversible and irreversible componentsArthritis Rheum 2006 54(9):2784-92. [Google Scholar]
[10]. Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritisArthritis Rheum 1988 31(3):315-24. [Google Scholar]
[11]. Guisti G, Galanti B, Colorimetric Method. In: Bergmeyer HU, editorMethods of enzymatic analysis 1984 Verlag ChemieWeinheim:315-23. [Google Scholar]
[12]. Karsdal MA, Woodworth T, Henriksen K, Maksymowych WP, Genant H, Vergnaud P, Biochemical markers of ongoing joint damage in rheumatoid arthritis–current and future applications, limitations and opportunitiesArthritis Res Ther 2011 13(2):215 [Google Scholar]
[13]. Matsui T, Kuga Y, Kaneko A, Nishino J, Eto Y, Chiba N, Disease Activity Score 28 (DAS28) using C-reactive protein underestimates disease activity and overestimates EULAR response criteria compared with DAS28 using erythrocyte sedimentation rate in a large observational cohort of rheumatoid arthritis patients in JapanAnn Rheum Dis 2007 66(9):1221-6. [Google Scholar]
[14]. Klimek E, Skalska A, Kwasny-Krochin B, Surdacki A, Sulicka J, Korkosz M, Differential associations of inflammatory and endothelial biomarkers with disease activity in rheumatoid arthritis of short durationMediators Inflamm 2014 2014:681635 [Google Scholar]
[15]. Hitoglou S, Hatzistilianou M, Gougoustamou D, Athanassiadou F, Kotsis A, Catriu D, Adenosine deaminase activity and its isoenzyme pattern in patients with juvenile rheumatoid arthritis and systemic lupus erythematosusClin Rheumatol 2001 20(6):411-16. [Google Scholar]
[16]. Nalesnik M, Nikolic JM, Jandric S, Adenosine deaminase and C-reactive protein in diagnosing and monitoring of rheumatoid arthritisMed Glas (Zenica) 2011 8(1):163-68. [Google Scholar]
[17]. Yuksel H, Akoglu TF, Serum and synovial fluid adenosine deaminase activity in patients with rheumatoid arthritis, osteoarthritis, and reactive arthritisAnn Rheum Dis 1988 47(6):492-95. [Google Scholar]
[18]. Sari RA, Taysi S, Yilmaz O, Bakan N, Correlation of serum levels of adenosine deaminase activity and its isoenzymes with disease activity in rheumatoid arthritisClin Exp Rheumatol 2003 21(1):87-90. [Google Scholar]
[19]. Haque SS, Kumar S, Kumari R, Kumar U, Saran A, Tanweeruddin M, Evaluation of Biochemical marker for the diagnosis of Rheumatoid arthritisJ Health Sciences 2014 04(01):187-92. [Google Scholar]
[20]. Haskó G, Cronstein B, Regulation of inflammation by adenosineFront Immunol 2013 4:85 [Google Scholar]
[21]. Zamani B, Jamali R, Jamali A, Serum adenosine deaminase may predict disease activity in rheumatoid arthritisRheumatol Int 2012 32(7):1967-75. [Google Scholar]
[22]. Bakker MF, Cavet G, Jacobs JW, Bijlsma JW, Haney DJ, Shen Y, Performance of a multi-biomarker score measuring rheumatoid arthritis disease activity in the CAMERA tight control studyAnn Rheum Dis 2012 71(10):1692-97. [Google Scholar]
[23]. Zakeri Z, Izadi S, Niazi A, Bari Z, Zendeboodi S, Shakiba M, Comparison of adenosine deaminase levels in serum and synovial fluid between patients with rheumatoid arthritis and osteoarthritisInt J Clin Exp Med 2012 5(2):195-200. [Google Scholar]