Planning for proper postoperative pain management is an essential component of good anaesthetic practice since the consequences of untreated pain can be devastating [1]. Adequate analgesia aids to restore normal functions including ventilation, coughing and mobility, thereby facilitating early rehabilitation and shortened hospital stay [2].
Until recently, bupivacaine has been the most commonly used drug for postoperative epidural analgesia. Despite a reasonable safety profile, it is being replaced with ropivacaine, a novel, pure levorotatory, safe long-acting local anaesthetic. The well-known toxic effects of bupivacaine on the central nervous system and the cardiovascular system are less severe with ropivacaine at comparable plasma levels [5]. It has been claimed that ropivacaine produces less motor block but equivalent analgesia compared with bupivacaine in similar doses although this is controversial [6].
Recently, few studies have been performed comparing bupivacaine with ropivacaine for postoperative analgesia [7], however, most of these studies have been in the setting of labour analgesia and the results have been contradictory [8]. With this background, we planned to study the postoperative analgesia of two concentrations of bupivacaine with two different doses of ropivacaine.
Materials and Methods
The study was conducted in a randomized, double blind manner in a tertiary level hospital after institutional ethics committee approval and informed patient consent. A total of 100 adult patients of either sex, belonging to ASA Grade I and II, scheduled to undergo lower limb surgery under combined spinal - epidural anaesthesia, were randomly allocated into either of four study groups of 25 patients each as per computer generated random number list. Intraoperative anaesthetic technique was same and standardized for all the four group of patients. Depending on the groups they were allocated, patients were started on postoperative epidural infusions after completion of surgery and skin dressing in the operating room itself, but beore shifting to post anaesthesia care unit (PACU).
Group I patients were started on epidural infusion of ropivacaine 0.2 %, Group II patients received epidural infusion of a solution of ropivacaine 0.1 % with fentanyl 2μg/ml, Group III was administered epidural infusion of bupivacaine 0.2 % and Group IV was administered solution of bupivacaine 0.1 % with fentanyl 2μg/ml.
Patients with a history of significant coexisting diseases like ischaemic heart disease, impaired renal functions and severe liver disease, any contraindication to regional anaesthesia, patients with a history of anaphylaxis to local anaesthetics and allergy to the drugs to be used, patients with a history of diseases predisposing to altered sensation like diabetes mellitus & neuropathies, patients with spine deformities, morbidly obese patients and patients with atrioventricular block, incomplete or partial heart blocks were excluded from the study.
All patients were examined a day prior to surgery and were pre-medicated with Tab. alrpazolam 0.5 mg and Tab. ranitidine 150 mg night prior to surgery.
On the day of surgery, patients were shifted to operation theatre and baseline parameters like Heart Rate (HR), Systolic Blood Pressure (SBP), Diastolic Blood Pressure (DBP), Respiratory Rate (RR), Peripheral Oxygen Saturation (SpO2) and Electrocardiography (ECG) were recorded. An intravenous access was achieved and preloading done with 10 ml/kg of Normal Saline solution over 20 minutes. All patients were administered combined spinal epidural block under all aseptic precautions in the sitting position. A 16G Tuohy’s needle was inserted after local infiltration at L2-L3 Lumber interspace using loss of resistance technique. Epidural catheter (16G) was inserted 4-5 cm in the epidural space and secured with antiseptic dressing. A test dose of 3ml of 2% Xylocaine with 1:200,000 epinephrine was given. A 26G Quincke’s spinal needle was introduced in the subarachnoid space at L3-L4 and 0.5% Bupivacaine 12.5mg was introduced into the subarachnoid space. Surgery was allowed to commence on achieving adequate sensory block height (T9-10). Patients were continuously monitored for HR, RR, SpO2, SBP, and DBP.
On completion of surgery and skin dressing, all haemodynamic parameters (HR, SBP, DBP, RR, and SpO2), sensory, motor block and VAS score were recorded and postoperative epidural infusion started as per the group allocation, after a 5 ml bolus of drug before shifting the patient to PACU. This time was labeled as time ‘E’ and all subsequent recordings were made from this time. The solution for the epidural infusion was prepared by an independent consultant anaesthesiologist who was not participating in the study and was coded for the purpose of the study. The solutions were decoded at the end of the study. The observer anaesthesiologist and the patient were also unaware of the group allocation. The epidural infusion was started using a syringe pump and 50ml solution in 50ml syringe (time E). All subsequent observations were recorded from this time onwards. All infusions were started at the rate of 6ml/h in a syringe pump. A supplemental bolus dose of 2 ml of epidural infusion drug was given, whenever the VAS score was greater than 3 or the patient requested for an additional analgesic. An interval of 10 minutes was enforced between two supplemental bolus doses of epidural infusion and a maximum of 3 bolus doses were allowed per hour. In case of insufficient analgesia (VAS >3 after two consecutive epidural boluses), rescue analgesia with injection (Inj) of Tramadol 50 mg slowly was administered intravenously (IV). Patients were shifted to postoperative recovery room under strict monitoring after 20 min of epidural bolus dose. All patients received oxygen supplementation by venturi face mask@ 6L/min with FiO2-0.5. All observations were recorded by an anaesthesiologist who was blinded to the group allocation of the patient.
SBP, DBP, HR, RR, SpO2, sensory, motor block and VAS score were recorded 5 mins before the beginning and 5 mins after giving of the epidural bolus.
At the start of the epidural infusion all haemodynamic parameters (HR, SBP, DBP, RR, SpO2), level of sensory & motor block and VAS score were recorded (time E). Subsequent recordings were made at 5, 10, 20, 30 mins and thereafter every half hourly till four hours and after that every four hourly till 24 hours. If SBP was less than 30% of baseline value or 90 mmHg, intravenous (i.v.) crystalloids 200- 300 ml were given along with Inj. Mephentermine, 3-6 mg IV, which could be repeated as per requirement. If HR was less than 50 beats/min, 0.5 mg of atropine sulphate was administered intravenously. Side effects like nausea, vomiting, itching, pruritis, hypotension; bradycardia, sedation were recorded. A rescue antiemetic in the form of inj of ondansetron hydrochloride 4 mg IV stat was given when patient had nausea score of ≥ 3. Nausea was defined as the subjective sensation of a desire to vomit without any expulsive muscular movements. Vomiting was defined as expulsive efforts followed by expulsion of gastric contents. The nausea score is categorized as 1-no nausea, 2- mild nausea, treatment is not necessary, 3- moderate nausea, treatment may be desirable, but patient can tolerate it, 4- severe nausea and treatment is necessary, 5- patient complains of urge to vomit despite treatment [9]. Sedation was assessed using the Ramsay Sedative assessment scale [10].
Quality of postoperative analgesia was assessed using Visual Analogue Scale, which was taken in the form patient marking on a 10 cm line having verbal anchors at both the ends. Mark 0 corresponds to ‘no pain’ and mark 10 corresponds to the ‘worst imaginable pain.’ Recording was done at 5, 10, 20, 30mins and thereafter every half hourly till fourth hour and then every four hours till completion of 24 hours [11].
Analgesic efficacy was assessed using total rescue analgesic consumption and Patient Satisfaction Scale was used to assess the overall satisfaction level of patient to the postoperative analgesia provided. Wherein, score of 0 means No satisfaction, 1- Partially satisfied, 2- Fully satisfied, 3-Fully satisfied and would recommend this analgesic technique to others [12].
At the end of 24 hour observation period, the epidural infusion was discontinued and patient was observed by the anaesthesiologists for another period of 24 hours during which patient was started on oral analgesic at the discretion of the treating physician. The epidural catheter was removed using all aseptic precautions and an antiseptic dressing applied and care provided as per hospital protocol.
(As per the protocol in our hospital, patients are kept in PACU only for 24 hours after which they are shifted to Wards and patients are not sent to wards with epidural catheters in situ to avoid any untoward complications).
Statistical Analysis
Based on a previous study, a sample size of 23 patients for each group was needed to detect 10% difference with 90% power and α of 0.05 [13]. After completion of the study, observations obtained were tabulated and analysed using appropriate statistical methods. Paired & unpaired Student t-test were used for demographic data and ANOVA was used for repetitive observations among inter & intragroup comparisons.
Results
All the groups were comparable with regards to mean age, sex distribution and the duration of surgery [Table/Fig-1].
| Mean Age(Years) | Male:Female ratio | Duration of Surgery (Min) |
|---|
| Group I | 54.72 | 15:10(60%:40%) | 112.80 |
| Group II | 54.92 | 17:8(68%:32%) | 111.60 |
| Group III | 56.52 | 12:13(48%:52%) | 114.80 |
| Group IV | 52.32 | 12:13(48%:52%) | 110.40 |
| Total | 54.62 | 56:44(56%:44%) | 112.40 |
| p-value | 0.836 | 0.404 | 0.862 |
Haemodynamic Variables
Heart Rate
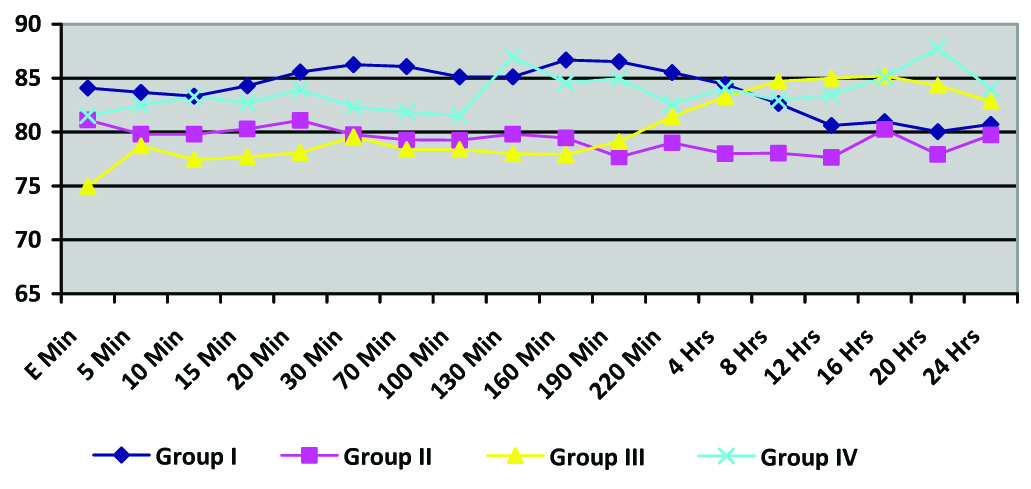
Mean baseline HR was similar in all the four groups and there were no significant changes from the baseline with time in any of the four groups. There was no statistically significant difference in postoperative mean heart rate in any of the four groups (p-value=0.414) [Table/Fig-2].
Heart Rate (Intergroup comparisons were done between the four groups; p-value > 0.05 at all time intervals)
(E time is the time when epidural infusion was started and subsequent times were measured from thereon)
Mean Arterial Blood Pressure
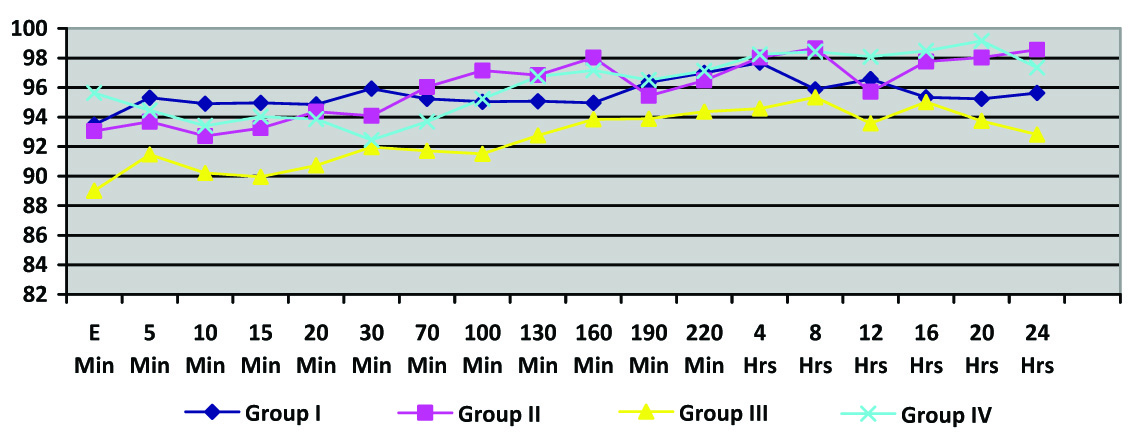
Mean blood pressure was also similar in all the groups and there was no statistically significant change from baseline in any of the four groups. (p-value 0.191) [Table/Fig-3].
Mean Arterial Blood Pressure
(Intergroup comparisons were done between the four groups; p-value > 0.05 at all time intervals)
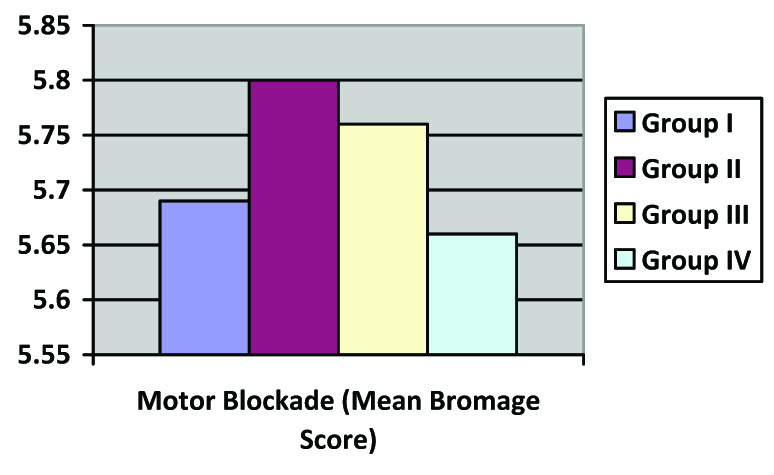
Four percent each in group I and II and 8% in group IV had mild nausea for which no treatment was required. None of the patients in group III had nausea and vomiting. Two (8%) patients in group IV were observed to have grade I sedation. Three patients (12%) in group IV reported pruritis. Pruritis was not observed in any other groups. This difference in the incidence of side effects was statistically not significant in any of the groups [Table/Fig-4]. Motor blockade was also found to be similar in all the groups, there being no statistically significant (p-value=0.187) difference in postoperative modified bromage score in all the groups [Table/Fig-5a].
Incidence of adverse effects
| Incidence of Adverse effect (%age) | Group I | Group II | Group III | Group IV | p-value |
|---|
| Nausea/Vomiting | 4% | 4% | 0 | 8% | 0.508 |
| Sedation | 0 | 0 | 0 | 8% | 0.217 |
| Pruritus | 0 | 0 | 0 | 12% | 0.197 |
| Postoperative Motor blockade(Mean Modified Bromage score) | 5.69±0.25 | 5.80±0.26 | 5.76±0.26 | 5.66±0.33 | 0.157 |
Postoperative Mean Modified Bromage Score after starting Epidural infusion. (p-value for intergroup comparisons 0.187)
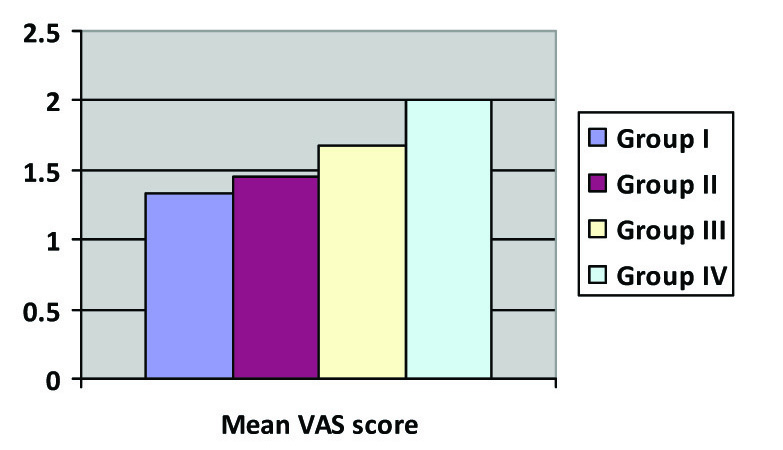
(b) Intergroup comparisons of Mean VAS; p-value = 0.286
(c) Intergroup comparisons of Total Epidural infusion administered abobe basal infusion; p-value < 0.001
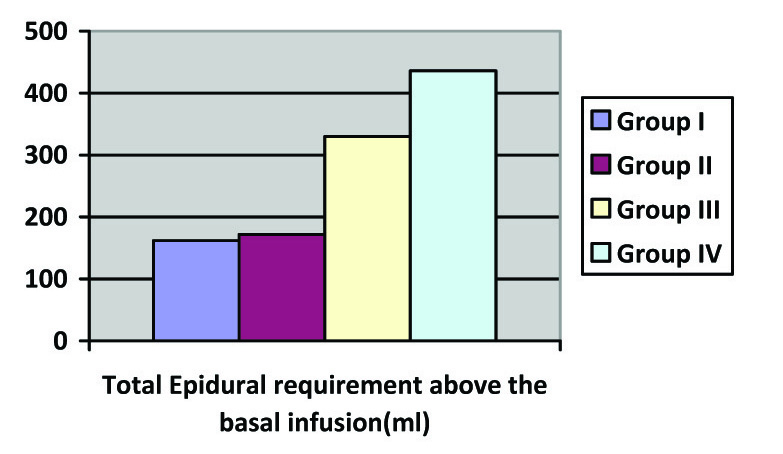
Mean VAS was similar among all the four groups for the first 100 minutes. However, at 130 min, 190 min, 4 hour, 8 hour, 12 hour and 20 hours, mean VAS was significantly higher in group IV as compared to other three Groups and it was least in group I, the difference being statistically significant (p-value 0.027). Mean VAS among Groups II and III were statistically similar at all time intervals. There was a significantly greater consumption of supplemental epidural boluses above the baseline with Group IV having the highest consumption of rescue epidural boluses and group I having the least consumption [Table/Fig-5b,c].
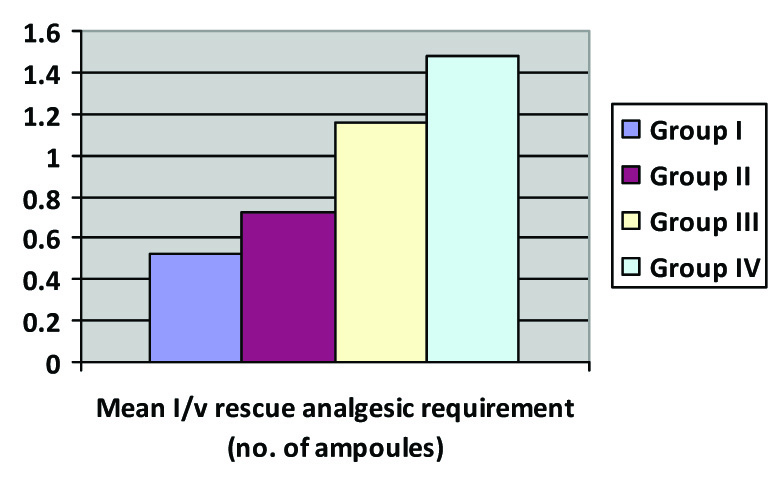
Thus groups III and IV required higher amounts of supplemental epidural boluses and the difference was statistically highly significant with consumption being highest in Group IV. In addition, there was a statistically significant difference in postoperative mean rescue IV analgesic consumption among all the Groups (p-value=0.005). The lowest demand for postoperative rescue IV analgesic was in Group I where 68 % subjects did not require any rescue analgesic. In Group II, III and IV 48, 36, 32 % subjects did not require any rescue analgesic. The mean total rescue IV analgesic dose received in group I was also least in Group I and highest in Group IV [Table/Fig-6].
Analgesic Efficacy
Intergroup comparison of Mean I/V Rescue analgesia; p-value = 0.016
Discussion
Ever since ropivacaine was introduced, there has been an interest to evaluate its safety and dose equivalence as compared to bupivacaine. It has been concluded from the Minimum Local Analgesic Concentration (MLAC) studies that 0.2% ropivacaine is the analgesic equivalent of 0.1% bupivacaine with fentanyl 2μg/ml [13]. A study comparing 0.125% ropivacaine and 0.125% bupivacaine as patient controlled epidural infusion observed no major differences between the two drugs in the form of their local anaesthetic/analgesic use, or even in terms of side effects [14]. Hence 0.125% ropivacaine and 0.125% bupivacaine have been found to be clinically indistinguishable for labor analgesia using patient-controlled epidural infusion technique. This formed the basis of using equivalent concentrations of epidural infusions in all groups of our study.
We observed that there was no statistically significant difference in mean HR, systolic BP, diastolic BP, mean MAP, RR and oxygen saturation at any time among all groups. These findings suggest that all the groups had adequate pain relief since uncontrolled pain leads to tachycardia and hypertension. Berti et al., reported similar observations in patients undergoing major abdominal surgeries while comparing a ropivacaine 0.2 % versus bupivacaine 0.125 % in combination with Fenatnyl 2 μg/ml [15].
Patients who received 0.2 % ropivacaine had the lowest VAS scores and least consumption of supplemental analgesics, signifying better analgesic effect. At the same time, motor blockade was lesser as compared to the patients who received bupivacaine. Jagtap et al., reported similar findings when comparing ropivacaine-fentanyl versus bupivacaine-fentanyl for intrathecal use in lower limb surgeries [16]. They observed that both groups had similar onset of sensory and motor block but motor blockade regressed earlier in the ropivacaine-fentanyl group. These findings suggest that ropivacaine has decreased potency for motor block when compared to an equianalgesic concentration of bupivacaine for epidural use. Ropivacaine is less lipophilic than bupivacaine and is less likely to penetrate large myelinated motor fibres; therefore, it has selective action on the pain-transmitting Aβ and C nerves rather than Aβ fibres, which are involved in motor function [17]. The decreased motor block with epidural ropivacaine confers an advantage in terms of early mobility over bupivacaine when the sensory block potency is approximately equivalent amongst the two groups. Rapid patient mobilization is an integral part of speedy recovery after lower limb surgery, and this leads to a decrease in duration of hospitalization by one to two days [18]. Our results are simiar to those of Berti et al., as they also reported higher supplemental analgesic consumption in the patients receiving combination of 0.125 % bupivacaine and 2 μg/ml fentanyl as compared to those who received 0.2 % ropivacaine [15]. Similarly, Kanai A et al., also reported least VAS scores in patients receiving 0.2% ropivacaine with 2.2μg/ml fentanyl in their study [19]. However, in their study, the addition of fentanyl to ropivacine reduced the VAS but in our study among the Groups II and IV, addition of fenatnyl did not result in superior analgesic efficacy as compared to Group I. The difference could be due to the fact that we used lower concentrations of ropivacaine and bupivacaine in group II and Group IV, whereas fentanyl (2.2μg/ml) was added to a 0.2 % ropivacine by Kanai et al., [19].
The incidence of adverse effects of nausea, vomitting, sedation and pruritis was similar in all the four groups. Our results were similar to those of Badner et al., who compared fentanyl and bupivacaine with fentanyl. They found no statistical significance in terms of nausea, vomiting and pruritis at any time of the epidural infusion [20].
Berti M et al., observed higher number of incremental doses and larger total volume of analgesic solution infused over 24 hours in the 0.125% bupivacaine with 2μg/ml fentanyl group [15]. We report similar findings with higher total mean epidural consumption, total epidural boluses and total rescue analgesic consumption in Group IV of our study.
In our study, the difference between total postoperative rescue analgesic consumption (IV tramadol) was statistically significant among all groups. The total rescue analgesic consumption was lowest in ropivacaine 0.2% which corresponds to lower VAS scores. bupivacaine 0.2% alone and 0.1% with fentanyl 2μg/ml had higher VAS scores as compared to ropivacaine 0.2% alone and 0.1%with fentanyl 2μg/ml, thus requiring frequent doses of rescue analgesics.
Limtations
Our study had few limtations. A larger sample size, assessment of degree of ambulation and a longer period of assessment of upto 48 hours would have helped us delineate further advantages of ropivacine –fentanyl epidural infusions for postoperative pain relief.
Conclusion
Thus we conclude that ropivacaine offers significantly superior postoperative analgesia both when either used alone or in combination with fentanyl. It also offers an advantage of fewer epidural and resuce analgesic dose consumption, with consequent negligible side effects. Hence it can be recommended as a safer choice of epidural local anaesthetic for postoperative analgesia following lower libmb surgeries.