Introduction
Rheumatoid Arthritis (RA) is a systemic autoimmune disease. It is associated with several auto antibodies which can serve as diagnostic and prognostic markers.
Aim
In this study, Anti perinuclear Factor (APF) was evaluated as a biomarker in comparison with Rheumatoid Factor (RF) in Rheumatoid Arthritis.
Materials and Methods
Fifty two sera of patients with RA (mean age 48±15.8), 23 sera of Patient control group (mean age 32.5 ± 16.9) and 30 sera of Healthy control group (mean age 32.1± 16.9) were analysed. The method is based on the binding of APF to perinuclear keratohyalin granules of buccal mucosal cell and its detection using a fluorescently labeled anti human total antiserum.
Results:
APF were found in 71.2 %(37/52) of patients with RA. The sensitivity and specificity for APF from 1/5 serum dilution was 71.2% and 94.3% respectively. RF test had higher sensitivity (88.5%) compare to the APF test (71.2%), but its specificity was (86.8%) less than APF (94.3%).
There was no significant relationship between the onset of APF and severity of disease but there was significant relationship between the APF titer and severity of disease (p<0.05).
Conclusion
It is concluded that APF test is a valuable serological tool for the diagnosis of the disease and a useful serological marker to differentiate from the other inflammatory rheumatoid diseases.
Autoimmunity, Biomarker, Keratohyaline granules, Rheumatoid factor
Introduction
Rheumatoid Arthritis (RA) is a type of autoimmune rheumatoid and systemic disease which affect about 1% of world’s population [1]. Auto antibodies are discovered in this disease, with unknown role of pathogenecity but are valuable in diagnosis, prognosis and evaluation of the severity of the disease.
The most common type of such antibodies is Rheumatoid Factor (RF) inspite of high sensitivity of RF, it is not specific for diagnosis. Hence, the efficiency of other serological marker for rheumatoid arthritis would be beneficial. One of these serological markers is Anti perinuclear Factor (APF). This antibody is adjacent to the granules present in the mucosal cells of human (Keratohyalin granules).
Presence of this antibody (ab) has been described by Nienhuis and Mandema in 1964, which showed its specificity for RA. Saraux and et al., compared the diagnostic values of anti perinuclear factor (APF), antikeratin antibody (AKA) and anti-cyclic citrullinated peptides (anti-CCP) to discriminate between patients with and without rheumatoid arthritis (RA) [2]. Jong-Moon Choi and et al., believed that antiperinuclear factor (APF) test, an originator of ACPA, is highly specific for rheumatoid arthritis and can be detected in RF or anti-cyclic citrullinated peptide (anti-CCP) negative rheumatoid arthritis [3].
Duck-An Kim et al., detected performing APF test along with anti-CCP test gives great help in the diagnosis of RA [4].
Generally, IgG class of AB is important [5,6]. Due to sensitivity of (36 - 87%) [7–12] and high specificity of (73-100%) [10,11] is a valuable serological tool for the diagnosis of the disease [7,10] and a useful serological marker to differentiate this disease from the other inflammatory rheumatoid diseases [9].
Antibody is also found in the seronegative RA patients. This AB is independent to the age, sex [11] and the duration of disease [6] and is appeared soon and even may appear before the onset of clinically rheumatoid arthritis [13–15] and in relation to the severity of disease. Sensitivity of APF is the same degree of RF and its specificity is more than RF specificity in the diagnosis of RA [9,10] Presence of APF can be detected only by the immunofluorescence technique.
This Ab binds to cytoplasmic granules in human buccal mucosal cells and on the basis of their histologic similarity with keratohyaline bodies in the stratum granulosum of human epidermis, known as keratohyalin granules [12]. It is an insoluble protein sensitive to freezing and its antingenicity reduces the different methods of fixation.
Since APF remains in the rheumatoid nodules and synovial fluid [10] and is related to the presence of RF [6,11]. APF is helpful in the diagnosis of early RA and can be considered as basic criteria for classification of RA [16–19].
So, in this study we evaluated APF as a diagnostic marker in comparison with RF for rheumatoid arthritis.
Materials and Methods
Sampling were done by non-probability and easy method. The study was performed according to the American College of Rheumatology (ACR) on 52 definite Rheumatoid Arthritis patients with mean age 48±15.8 years. A questionnaire comprising the questions relevant to the diagnosis of the disease was prepared by the rheumatologist. The questionnaire was based on sex, age, severity of disease, functional capacity and extra articular features, including (cutaneous vascolitis, ophtalmic, pulmonary -cardiac, renal digestive -spleenomegaly and adenomegaly).
The disease severity was categorized into three degree: of mild, moderate and severe. Also, the functional capacity of RA was classified into four groups: 1) normal; 2) moderate; 3) severe disorder; 4) lack of movement power [20].
The patient control group (patients with rheumatoid diseases and non rheumatoid arthritis) were 23 with mean age of 32.5±16.9 years and the healthy control groups were 30 with mean age of 32.1±16.9 years who were investigated for APF titer and RA-latex. Peripheral blood were collected, serum separated and preserved at -200C for testing. The samples were collected during 4 months and the study was carried out from Apr 2012 to Jun 2013.
Method of APF - IFA test
A Preparation of antigenic substrate
Samples of buccal mucosal epithelial cells were taken using a sterile foam plastic sponge after mouth washing of the donor. They were washed twice with phosphate buffer saline (PBS, pH 7.4), once with PT buffer (0.5 % Triton -X100 in PBS) and once again with PBS. Slides of cell suspension were prepared for PAS staining for the presence of positive granules cells. (At least 20% of the cells should be positive) after placing of cells suspension on the fluorescence slide and air-dried were stored for 2 weeks at -70°c.
B The Stage of APF -IFA test procedure
The slides with buccal mucosal cells were incubated with 10λ serum in 100% humid atmospheres for 90 minutes. The preparations were washed in PBS (3 ×10 min) and incubated with 10λ antihuman conjugated to FITC in dark for 30 min. After washing with PBS (3 ×10 min) the preparations were mounted in a glycerol/PBS (1/1) solution. The slides were studied under fluorescence microscope.
Statistical Analysis
Statistical analyses were performed using SPSS version 19. For description of the sample characteristic. The descriptive statistic such as frequency distribution, mean and standard deviation were used. The Statistical analysis of Tau B Kendall was used to determine the relation between the APF titer and severity of disease, functional capacity and extra articular features. The pearson correlation coefficient was used for determining the relation between the APF titer with the age, onset of disease and disease duration. The Chi-square test was used to determine between onset of APF with sex and RF in RA. The confidence index of 95% at the significant level of < 0.05 was used.
Results
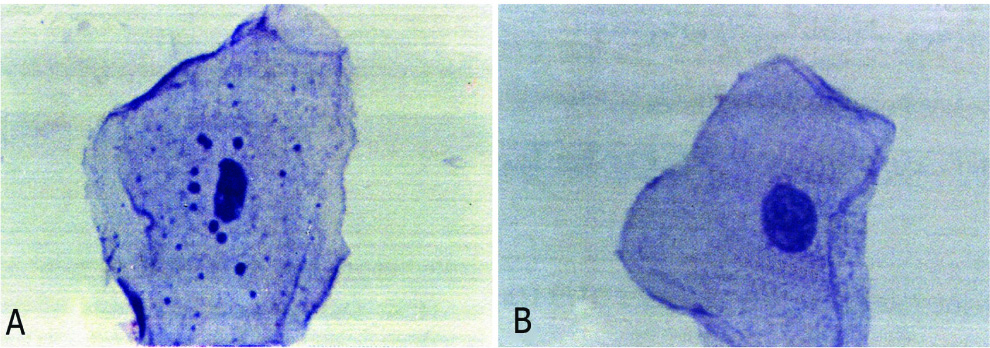
Human buccal epithelial cells with and without perinuclear keratohyalin granule cells were seen in PAS Staining in [Table/Fig-1a,b].
a) Epithelial cell with granule cells. b) Epithelial cell without granule cells.
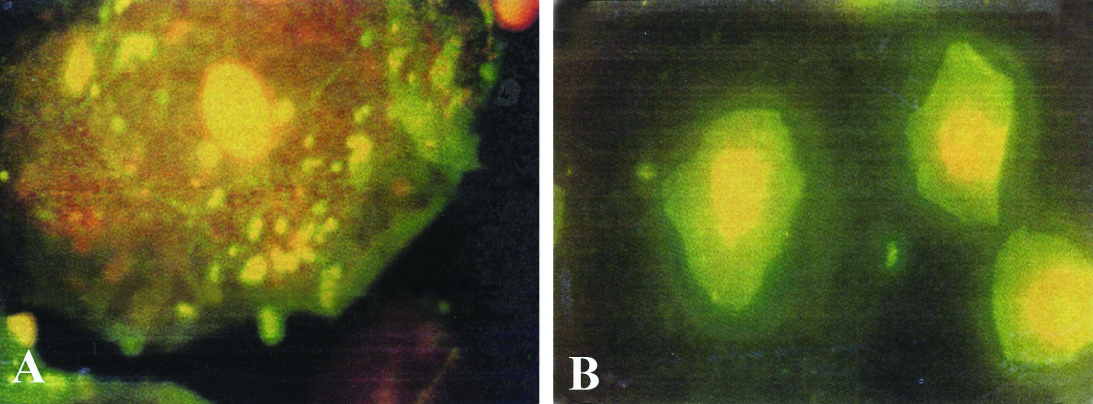
IIF (Indirect Immunofluorescence) was tested on human buccal epithelial cells for their APF activity. They revealed staining of the perinuclear granule cells in human buccal epithelial cells and were seen by fluorescent microscope in [Table/Fig-2a,b].
a) Epithelial cell with granule cells and positive APF activity. b) Epithelial cell with granule cells and negative APF activity
Frequency distribution of individuals was divided on the basis of sex in three groups of RA patients, patient control and healthy control. On the basis of results, there was no significant relationship between the groups and sex (p=0.126) [Table/Fig-3].
Frequency distribution of individuals on the basis of Age, sex and APF test in three groups
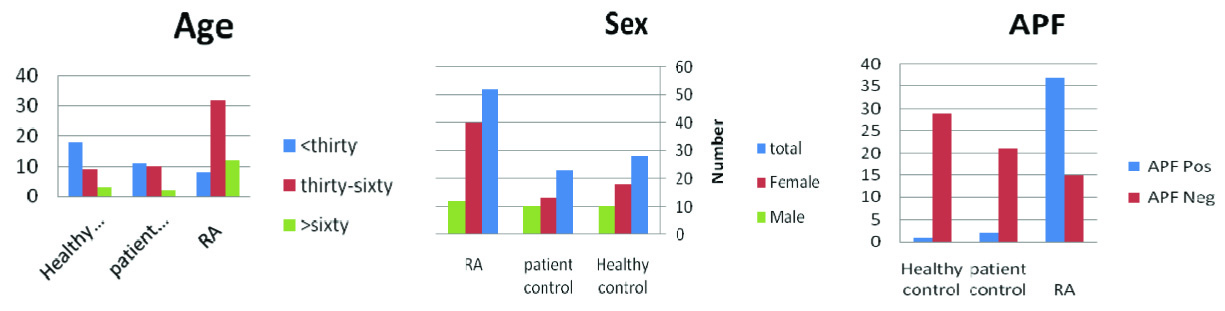
The mean age for the groups of RA, patient control and healthy control was 48.0±15.8, 32.5±16.9 and 32.1±16.9 respectively. There was statistically significant difference among three groups (p < 0.05). Also, the groups were divided into three subgroups, under 30, between 30-60 and more than 60-year-old. In RA group, most of the patients were between 30-60 years (61.5%). On the basis of λ2 test, the groups were not matched for age (p< 0.05) [Table/Fig-3].
On the basis of λ2 test, there was significant relationship between the groups and the presence of APF and RA (p<0.0001) [Table/Fig-3].
Comparing the sensitivity and specificity of APF test for different titers in RA, in 1/5 titer, the sensitivity and specificity of this test was 71.2% and 94.3% respectively. In the next titers, though specificity of the test increases, but the sensitivity decreases. Hence, we recommend the 1/5 titer as a cut off or least significant titer in the diagnosis or confirmation of RA disease [Table/Fig-4].
Comparison of the sensitivity and specificity of APF test for different titers in RA
| titer | specificity (%) | sensitivity (%) | Control groups | Rheumatoid Arthritis |
|---|
| positive cases | positive cases |
|---|
| 1/51/101/201/401/801/1601/3201/6401/1280 | 94.394.398.198.198.1100100100100 | 71.257.734.62515.49.63.93.91.9 | 33111______ | 3730181385221 |
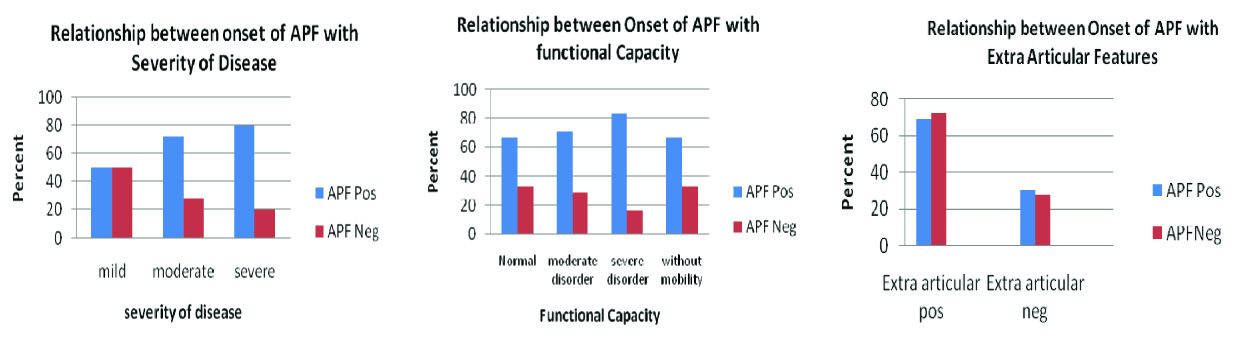
In relation onset of APF and severity of disease there was no significant relationship (p= 0.311) Also, in relation onset of APF and functional capacity, no significant association was served [Table/Fig-5]. Regarding to the relevance on the onset of APF and APF titer with extra articular features in the RA patients, there was no significant relationship [Table/Fig-5].
Disease Features of RA with onset of APF
There was significant relationship between the APF titer and severity of disease (p<0.05) [Table/Fig-6].
Disease features of RA with APF titer
| Disease features | RA cases (%) | APF titer | p-value |
|---|
| severity of disease |
| mild | 8(15.4) | 0-1/10 | 0.05 (S) |
| moderate | 29 (55.8) | 0–1/640 | 0.05 (S) |
| severe | 15 (28.8) | 0–1/1280 | 0.05 (S) |
| functional capacity |
| I | 12 (23.1) | 0–1/80 | 0.131 (NS) |
| II | 31 (59.6) | 0–1/640 | 0.131 (NS) |
| III | 6 (11.5) | 0–1/20 | 0.131 (NS) |
| Iς | 3 (5.8) | 0–1/1280 | |
| extra articular features |
| positive | 23 (44.2) | 0–1/160 | 0.290 (NS) |
| negative | 29 (55.8) | 0–1/1280 | 0.290 (NS) |
Regarding the relevance on the onset of APF with sex in the RA patients, there was no significant relationship (p=0.451) [Table/Fig-7].
Relationship between onset of APF and sex in RA patients
| APFSex | Negative | Positive | Total | p-value |
|---|
| Number of cases (%) | Number of cases (%) |
| FemaleMale | 10 (66.7)5 (33.3) | 30 (81)7 (19) | 4012 | 0.451 |
| Total | 15 | 37 | 52 |
On the basis of λ2 test there was significant relationship between the RF and RA (p<0.0001) [Table/Fig-8].
Relationship between onset of RF and RA
| Group→ | RA cases (%) | Patient control (%) | Healthy control (%) | Total (%) |
|---|
| RF↓ |
|---|
| Negative | 6 (11.5) | 18 (78.3) | 27 (90) | 51 (48.6) |
| +1 | 4 (7.7) | 3 (13.04) | 2(6.7) | 9 (8.6) |
| +2 | 13(25) | 1 (4.34) | 1 (3.3) | 15 (14.3) |
| +3 | 22(42.3) | 1 (4.34) | 0(0) | 23(21.9) |
| +4 | 7(13.5) | 0(0) | 0(0) | 7(6.7) |
| Total | 52 (49.5) | 23 (21.9) | 30 (28.6) | 105 (100) |
On evaluation of indexes relevant to the credit of tests such as APF-IFA and RF-latex in the diagnosis of RA, RF test had higher sensitivity (88.5%) compare to the APF test (71.2%), but its specificity was (86.8%) less than APF (94.3%) [Table/Fig-9].
Comparison of the sensitivity and specificity of APF test and RF test
| Test | Sensitivity (%) | Specificity (%) |
|---|
| APF | 71.2% | 94.3% |
| RF | 88.5% | 86.8% |
Discussion
In this research, we compared APF ab as diagnostic marker in comparison with RA-latex in RA.
In our study, the ratio of female to male was 40/12=3.3 in adaption with others [21].
In the RA patients, most of them were in the age group of 30-60 years [Table/Fig-3] and the other studies indicate the same [21].
On the basis of the sensitivity and specificity of APF test, we considered the titer 1/5 as a cut off or least significant titer in RA patients [Table/Fig-4]. This dilution was the most proper proposed titer for APF test. Also, in the performed studies APF were observed in RA patients with sensitivity 36-87% [7–12] and specificity 73-100% [9–11]. Furthermore, APF was a valuable serological tool for the diagnosis of RA disease and a useful serological marker to differentiate of disease from the other rheumatic disease (p=0.05) [Table/Fig-4] [9,10].
In this study, no significant relationship was found between the onset of APF and severity of disease in RA patients. It means that APF appeared in the patients with both the mild and moderate forms of disease [Table/Fig-5] but there was a significant relationship between APF titer and severity of disease. In addition, in the mild form of the disease the APF titre was not high, but the moderate and severe form of disease showed a high titre of APF (p=0.05) [Table/Fig-6]. Other studies also indicated the same [18,20].
Also, no statistically significant relationship was found between the onset of APF and the functional capacity of RA patients under study [Table/Fig-5]. The functional capacity was classified into four classes: 1) normal; 2) moderate; 3) severe disorder; 4) lack of movement power [20]. Referring to the results of the statistical tests and [Table/Fig-5], it can be concluded that APF was present in all four classes of the functional capacity. The results of the statistical tests about the APF titer in relation to the functional capacity of the patients [Table/Fig-6], there were no significant differences between functional capacity groups. These results were consistent with other studies too [10].
Also, in the RA patients there was no significant relation between the onset of APF and APF titer with the extra articular features [Table/Figure-4,6]. But, another study indicated an existent relationship between the APF titer and extra articular features [Table/Fig-6] [5].
In addition, the statistical analysis indicated a significant relationship between the RF and RA (p<0.0001) [Table/Fig-8]. In this evaluation, the indexes relevant to reliability of APF -IFA and RF-latex in diagnosis of the disease, RF-test had higher sensitivity (88.5%) compared to the APF test (71.2%), but its specificity (86.8%) was lower than APF test (94.3%). It indicated that APF-IFA compare to RF-latex test was more specific and had a median value in the diagnosis and high credit for the confirmation of the disease [Table/Fig-9]. In addition, the other study had shown that RF-latex test had the highest sensitivity with least specificity [18]. Though, RA was observed in the higher age group (30-50 years) and occurred 2-3 times more in females, but no statistically significant difference was observed between the APF titer with the sex [Table/Fig-7] [11].
Conclusion
It can be concluded that APF test is a valuable serological tool for the diagnosis of the disease and a useful serological marker to differentiate this disease from the other inflammatory rheumatoid diseases.
[1]. Pavai S, Sargunan S, Zain AA, Chow SK, Analytical and diagnostic performance of an automated anti-CCP assayMalays J Pathol 2011 33(2):101-06. [Google Scholar]
[2]. Saraux A, Berthelot J, Mevauchelle V, Bendaoud B, Chales G, Value of antibodies to citrulline-containing peptides for diagnosing early rheumatoid arthritisJ Rheumatol 2003 30(12):2535-39. [Google Scholar]
[3]. Jong-Moon C, Ink-You K, A Preliminary Study for Applying Antiperinuclear Antibody Test to 2010 ACR/EULAR Classification Criteria for Rheumatoid ArthritisLab Med Online 2013 3(1):29-33. [Google Scholar]
[4]. Kim DA, TY Kim, Variable association of anti-CCP positivity with serum ferritin may be corrected by APF testRheumatol Int 2010 30(7):997-98. [Google Scholar]
[5]. Munoz-Fernandez S, Alvarez-Doforno R, Cuesta M, Balsa A, Fontan G, Antiperinuclear factor: a useful test for the diagnosis of rheumatoid arthritisRheumatol Int 1995 15(4):145-49. [Google Scholar]
[6]. Youinou P, Le Goff P, Dumay A, Lelong A, Fauquert P, The antiperinuclear factor. I. Clinical and serologic associationsClin Exp Rheumatol 1990 8(3):259-64. [Google Scholar]
[7]. Zhao J, Liu X, Wang Z, Liu R, Li Z, Is it necessary to combine detection of anticitrullinated protein antibodies in the diagnosis of rheumatoid arthritis?J Rheumatol 2010 37(12):2462-65. [Google Scholar]
[8]. Hoet RM, Voorsmit RA, Van Venrooij WJ, The perinuclear factor, a rheumatoid arthritis-specific autoantigen, is not present in keratohyalin granules of cultured buccal mucosa cellsClin Exp Immunol 1991 84(1):59-65. [Google Scholar]
[9]. Meyer O, Labarre C, Dougados M, Goupille P, Cantagrel A, Anticitrullinated protein/peptide antibody assays in early rheumatoid arthritis for predicting five year radiographic damageAnn Rheum Dis 2003 62(2):120-26. [Google Scholar]
[10]. Manera C, Franceschini F, Cretti L, Braga S, Cattanec R, Clinical heterogeneity of rheumatoid arthritis and the antiperinuclear factorJ Rheumatol 1994 21(11):2021-25. [Google Scholar]
[11]. Sebbag M, Simon M, Vincent C, Masson-Bessiere C, Girbal E, The antiperinuclear factor and the so-called antikeratin antibodies are the same rheumatoid arthritis-specific autoantibodiesJ Clin Invest 1995 95(6):2672-79. [Google Scholar]
[12]. Vivino FB, Maul GG, Histologic and electron microscopic characterization of the antiperinuclear factor antigenArthritis Rheum 1990 33(7):960-69. [Google Scholar]
[13]. Berthelot JM, Maugars Y, Castagne A, Audrain M, Prost A, Antiperinuclear factors are present in polyarthritis before ACR criteria for rheumatoid arthritis are fulfilledAnn Rheum Dis 1997 56(2):123-25. [Google Scholar]
[14]. Boerbooms AM, Antiperinuclear factors are present in polyarthritis before ACR criteria for rheumatoid arthritis are fulfilledAnn Rheum Dis 1997 56(6):395 [Google Scholar]
[15]. Cordonnie C, Meyer O, Palazzo E, de Bandt M, Elias A, Diagnostic value of anti-RA33 antibody, antikeratin antibody, antiperinuclear factor and antinuclear antibody in early rheumatoid arthritis: comparison with rheumatoid factorBr J Rheumatol 1996 35(7):620-24. [Google Scholar]
[16]. Boerbooms AM, Westgees AA, Reekers P, van de Putte LB, Immunogenetic heterogeneity of seronegative rheumatoid arthritis and the antiperinuclear factorAnn Rheum Dis 1990 49(1):15-17. [Google Scholar]
[17]. Aho K, von Essen R, Kurki P, Palosuo T, Heliovaara M, Antikeratin antibody and antiperinuclear factor as markers for subclinical rheumatoid disease processJ Rheumatol 1993 20(8):1278-81. [Google Scholar]
[18]. von Essen R, Kurki P, Isomaki H, Okubo S, Kautiainen H, Prospect for an additional laboratory criterion for rheumatoid arthritisScand J Rheumatol 1993 22(6):267-72. [Google Scholar]
[19]. Berthelot JM, Maugars Y, Audrain M, Castagne A, Prost A, Impact of using stored cells for immunofluorescence detection of antiperinuclear factor on sensitivity of the method for the diagnosis of rheumatoid arthritisRev Rhum Engl Ed 1995 62(7-8):507-12. [Google Scholar]
[20]. Hochberg MC, Chang RW, Dwosh I, Lindsey S, Pincus T, The American College of Rheumatology 1991 revised criteria for the classification of global functional status in rheumatoid arthritisArthritis Rheum 1992 35(5):498-502. [Google Scholar]
[21]. Diamond B, Systemic autoimmunity, in Fundamental immunology. WE. Paul, Editor. 2008 PhiladelphiaLippincott Williams & Wilkins:1313 [Google Scholar]