Primary Squamous Cell Carcinoma of Submandibular Salivary Gland with Sialo-Cutaneous Fistula: A Rare Case Report
Aditya Atul Kulkarni1, Sanjiv S. Thakur2
1 Senior Resident, Department of General Surgery, B.J. Government Medical College, Pune, India.
2 Professor and Head, Department of General Surgery, B.J. Government Medical College, Pune, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Aditya Atul Kulkarni, C/o Dr. Mankikar, 101, DSK Rohan, Near Model Colony Post Office, Model Colony, Shivajinagar, Pune- 16, India.
E-mail: draadityakulkarni@gmail.com
Malignant tumours of the submandibular salivary glands are rare entities. Most common malignant tumour of submandibular gland is mucoepidermoid carcinoma. Histological finding of squamous cell carcinoma is very rare in submandibular salivary gland. Metastasis from distant primary squamous malignancy, direct invasion from cutaneous or mucosal squamous carcinoma, squamous component of mucoepidermoid carcinoma or primary squamous cell carcinoma of salivary origin are some of the possible causes. Of these, the latter is distinctly uncommon. Primary squamous malignancy is diagnosed only after ruling out other possible explanations. A positive mucin stain in the tumour or synchronous/ metachronous squamous carcinoma elsewhere excludes the diagnosis of a primary carcinoma. Primary squamous carcinoma is seen most commonly in parotid gland and rarely in submandibular gland. We present a case of primary squamous cell carcinoma of right submandibular salivary gland in a 45-year old-man. This case is presented for the rare entity of primary squamous cell carcinoma in submandibular salivary gland.
Malignant tumours, Mandible, Neck dissection
Case Report
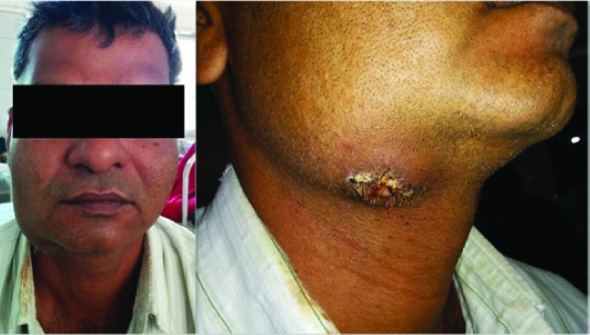
A 45-year-old male patient presented with a history of swelling in right submandibular region since 4 months. The swelling was painless and was gradually increasing in size. Since last two months, there was history of mucoid discharge from the swelling. The patient gave a history of smoking locally made bidi cigarettes for many years. On examination, there was a 5×7 cm swelling in right submandibular region [Table/Fig-1]. The swelling was firm to hard in consistency and was palpable on bidigital palpation. There was a fistula opening on the skin in the inferolateral aspect of the swelling. On manual pressure, scanty mucoid discharge was expressed from the fistula opening [Table/Fig-1]. On intraoral examination, the mucosa of oral cavity was normal. Apart from the lump, there were no other abnormal findings on oral, ear, nose and throat examination.
Photograph showing the swelling in right submandibular region with the fistula seen clearly
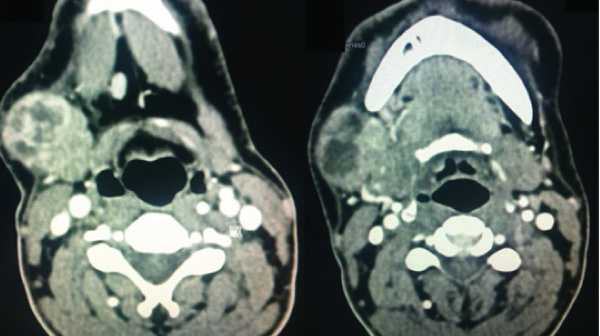
Ultrasonography of neck revealed a heterogeneous lesion involving right submandibular gland suggestive of neoplastic aetiology. CT scan of neck was suggestive of 4×3.8×3.6 cm sized enhancing mass lesion in right submandibular gland [Table/Fig-2&3]. There were multiple enlarged lymph nodes at level I, II and III on right side. Fine needle aspiration cytology (FNAC) from swelling was suspicious for squamous cell malignancy. To rule out synchronous malignancy, patient underwent screening upper endoscopy and video laryngoscopy, which were both normal.
Axial sections of CT scan showing an enhancing heterogenous mass in right submandibular region. Submandibular gland is not seen separately from the growth
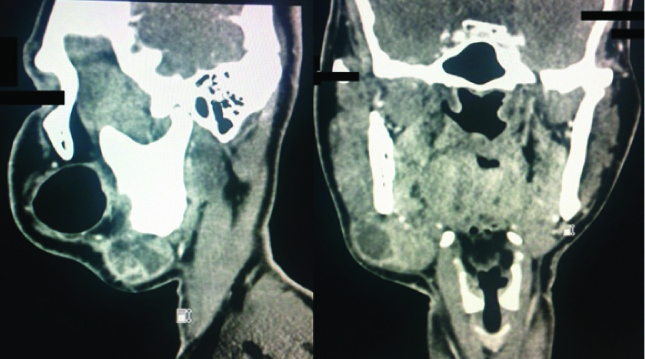
Coronal and sagittal sections of CT scan showing the tumour in right submandibular region
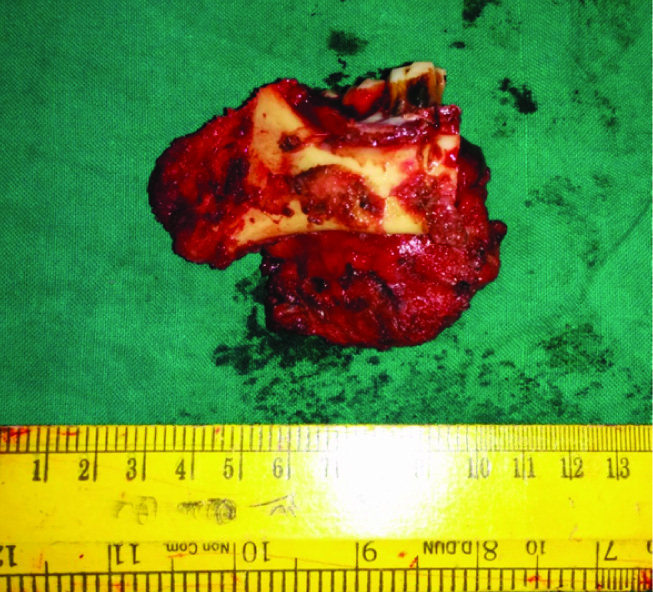
The patient was managed surgically. Intraoperatively, the growth was found to be adherent to the lower border of mandible. Frozen section of enlarged lymph nodes was positive for tumour. Hence, a radical excision comprising of submandibular gland with segmental mandibulectomy and ipsilateral modified radical neck dissection was performed [Table/Fig-4&5]. Reconstruction of the defect with flap closure was done. Postoperative course was uneventful.
Intraoperative photograph showing the completed radical excision with mandibulectomy and radical neck dissection
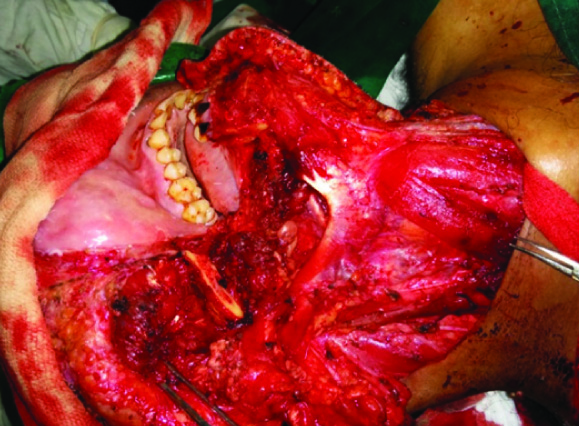
The resected specimen of submandibular tumour and segment of mandible adherent
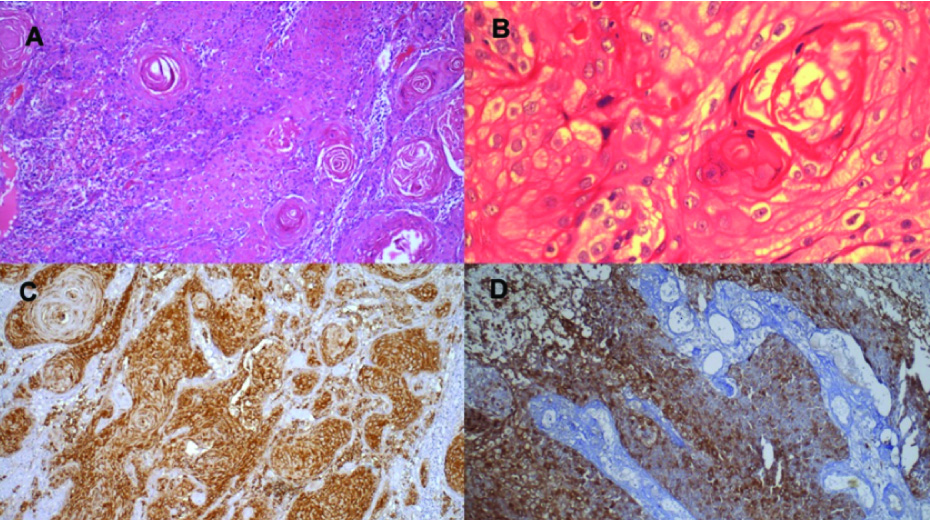
Histopathology revealed an infiltrating tumour composed of cells arranged in nests and solid sheets. The cells were round to polygonal with pleomorphic vesicular nuclei and eosinophilic cytoplasm. Foci of keratinization and pearls were seen. There was no evidence of lymphovascular invasion or perineural extension. Staining for mucin was negative. Immunohistochemistry showed positivity for cytokeratin and p63. Epithelial membrane antigen and S-100 were negative [Table/Fig-6a-d]. The diagnosis on microscopy was moderately differentiated squamous cell carcinoma. The margins of resection were clear of tumour. Neck dissection specimen showed that two lymph nodes out of sixteen were involved by tumour with evidence of extranodal invasion.
a) Photomicrograph showing keratin pearl in squamous cell carcinoma involving submandibular salivary gland (H&E stain, ×40); b) Photomicrograph showing keratin pearls (H&E stain, ×200); c) Cytokeratin positivity in squamous cell carcinoma in submandibular salivary gland (IHC stain, ×40); d) p63 positivity in squamous cell carcinoma in submandibular salivary gland (IHC stain, ×40)
As there was no other source of squamous cell carcinoma detected elsewhere in the patient, we made a final diagnosis of primary squamous cell carcinoma of submandibular salivary gland. Patient received adjuvant radiotherapy to the tumour area and the ipsilateral neck. Thereafter, he was kept on regular follow-up. Currently, the patient has no evidence of disease two years after diagnosis.
Discussion
Malignant tumours of submandibular salivary gland are uncommon. Around 50% of all submandibular tumours are malignant. Squamous cell carcinoma is one of the rarest of malignant salivary gland tumours. Batsakis et al., in a review of previous studies (a total of nearly 7000 salivary gland tumours) found squamous carcinoma in only 66 cases (0.9% of all cases) [1]. In a study of 498 major salivary gland carcinomas, 25 primary squamous cancers (approximately 5%) were found [2].
The commonest site for squamous carcinoma in major salivary gland is parotid gland. Primary squamous carcinoma accounts for 9% of all malignant parotid tumours and only 2% of malignant submandibular tumours [3]. A 30-year retrospective analysis of 50 patients with squamous cell carcinoma of the salivary glands found 42 patients with parotid tumours and 8 patients with submandibular tumours [4].
Primary squamous carcinoma of the salivary gland is a diagnosis of exclusion. If the patient has a history of or a synchronous squamous carcinoma, either in skin or mucosa, of the head and neck or in some distant site, the salivary gland involvement is regarded as metastatic. A prior history of irradiation is a strong risk factor for this tumour [2,4]. There is a strong male predilection and average age of patients is between 61 to 68 years. However, our patient was 45-year-old and gave no history of irradiation to head or neck region.
Usually, it is not possible to differentiate squamous carcinoma form other salivary gland malignancies on clinical or radiological examination. Malignant tumours are usually painful with short duration of symptoms. Fixation to the skin and deeper tissues may occur in some cases. Treatment includes radical resection of the involved submandibular salivary gland and surrounding tissues. Mandibular invasion is uncommon as the periosteum offers a natural barrier to spread of the tumour. Squamous carcinoma has a propensity for nodal metastasis, hence concurrent neck dissection is necessary, if cervical metastases are detected or suspected. Patients with cervical lymph node metastasis are at increased risk of distant metastasis. Postoperative adjuvant radiotherapy may be considered in view of the aggressive tumour behaviour, and there is some evidence that it may help to control local recurrence [5].
Gaikwad et al., reported a similar case with submandibular mass which on FNAC was diagnosed as squamous cell carcinoma [6]. The patient underwent wide excision and neck dissection for metastatic lymph nodes in neck. Manvikar et al., also reported a case similar to the present one where a 70-year-old female presented with rapidly growing submandibular swelling and was diagnosed as squamous cell carcinoma [7]. According to Rao et al., in a case report of submandibular squamous cell malignancy, the patient expired within 4 months of diagnosis due to pulmonary metastasis, highlighting the aggressive behaviour of these tumours [8]. Rasp et al., reported squamous cell carcinoma of the submandibular gland in a child aged 11 years and emphasized that risk of local recurrence and lymph node metastases is higher in children as compared to adult patients [9].
Taxy, in a retrospective review of salivary squamous tumours, concluded that “A primary malignant tumour that manifests squamous differentiation, or a metastasis, indicates an aggressive tumour that is unpredictably responsive to the major therapeutic modalities” [10]. Nearly 70% of patients present as stage III i.e. locally advanced disease. As per the literature, regional recurrence occurs in around 66% of cases within one year. Disease-free survival at 5 years is around 24% for parotid lesions and around 20% for submandibular gland tumours. Long-term follow-up is essential for all patients after therapy [11]. A point of note is that patients with metastatic squamous cell carcinoma and primary squamous cell carcinoma of submandibular salivary gland have a similar prognosis, regardless of treatment plan [10].
Conclusion
This case report highlights the rare entity of squamous cell carcinoma arising in major salivary (submandibular) gland. This entity must be considered in the differential diagnosis of swellings arising from salivary glands. These tumours usually have aggressive behaviour and hence multimodality therapy with surgery and radiation are necessary for ensuring good outcome.
[1]. Batsakis JG, McClatchey KD, Johns M, Regazi J, Primary squamous cell carcinoma of the parotid glandArch Otolaryngol 1976 102(6):355-57. [Google Scholar]
[2]. Spitz MR, Batsakis JG, Major salivary gland carcinoma: descriptive epidemiology and survival of 498 patientsArch Otolaryngol 1984 110(1):45-49. [Google Scholar]
[3]. Rosen J, Chapter 12: Major and minor salivary glands. In: Rosen J, editorAckerman’s surgical pathology 1996 Vol. 28th edChinaElseiver publications:639-73. [Google Scholar]
[4]. Shemen LJ, Huvos AG, Spiro RH, Squamous cell carcinoma of salivary gland originHead Neck Surg 1987 9(4):235-40. [Google Scholar]
[5]. Ying YL, Johnson JT, Myers EN, Squamous cell carcinoma of the parotid glandHead Neck 2006 28(7):626-32. [Google Scholar]
[6]. Gaikwad RV, Kumaraswamy SV, Keerthi R, Primary squamous cell carcinoma of the submandibular salivary gland: a rare entityJ Maxillofac Oral Surg 2015 14(Suppl 1):57-59. [Google Scholar]
[7]. Manvikar V, Ramulu S, Ravishanker ST, Chakravarthy C, Squamous cell carcinoma of submandibular salivary gland: A rare case reportJ Oral Maxillofac Pathol 2014 18:299-302. [Google Scholar]
[8]. Rao GM, Reddy Ranga SV, Janaki M, Reddy KL, Primary squamous cell carcinoma of the submandibular salivary glandIndian J Otolaryngol Head Neck Surg 2004 56(2):125-26. [Google Scholar]
[9]. Rasp G, Permanetter W, Malignant salivary gland tumors: squamous cell carcinoma of the submandibular gland in a childAm J Otolaryngol 1992 13(2):109-12. [Google Scholar]
[10]. Taxy JB, Squamous carcinoma in a major salivary gland: a review of the diagnostic considerationsArch Pathol Lab Med 2001 125(6):740-45. [Google Scholar]
[11]. Tran L, Sadeghi A, Hanson D, Juillard G, Mackintosh R, Calcaterra TC, Major salivary gland tumors: treatment results and prognostic factorsLaryngoscope 1986 96(10):1139-44. [Google Scholar]