In the arena of global health concern, cardiovascular problems pose a major threat to the society. The renaissance from balloon angioplasty to implantation of coronary stents has revolutionized the whole idea of safety and efficacy [1,2]. But, then again, the use of stents did not completely avoided restenosis and the frequency of restenosis persisted more than 30% in several subgroups [3–5]. This germinated the necessity for drug eluting stents (DES). First generation DES, with durable polymers, considerably negotiated the rates of revascularization as compared to bare metal stents (BMS) [6,7]. But, the utilization of non-biodegradable polymer in the first generation DES negatively affects its long term outcomes. The presence of polymer even after the drug has been eluted instigates local inflammatory reaction and delays healing of affected arteries [8]. This act as an Achilles heel and contributes to late thrombosis [9–11].
The use of biodegradable polymer in new generation DES enhances its long term safety and efficacy. The biodegradable polymer would soon degrade after drug has been eluted. This would reduce local inflammatory reaction and irritation leaving only a metal stent in adhesion with neointima and endothelium.
The Indolimus (Sahajanand Medical Technologies Pvt. Ltd., Surat, India) employs L605 cobalt chromium alloy as its stent platform. The metal stent is coated with biodegradable polymer to deliver sirolimus. The biodegradable polymer undergoes degradation in 9-12 months leaving only a metal stent along the arterial wall. Theoretically, it seems to improve arterial compliance and reduced need for prolonged antiplatelet therapy. But practically, there are still some queries relating to short and long-term safety and efficacy of sirolimus-eluting DES.
Thus, the main aim of this multi-centre, non-randomized, retrospective registry was to evaluate 9-months safety and efficacy of the Indolimus sirolimus-eluting stents in unselected consequent patients with complex coronary lesions.
Materials and Methods
Study Design
The INDOLIMUS-G is a multi-centre, non-randomized retrospective registry with a clear aim of evaluating safety and efficacy of Indolimus sirolimus-eluting stents in consecutive patients enrolled between April 2012 and May 2014. Various demographic, procedural and lesion characteristics and clinical follow-up data were collected for 1008 patients in whom study stent was implanted. The study was conducted in compliance with Declaration of Helsinki and was approved by institutional ethics committee. A signed informed consent was also obtained from each enrolled patients.
Study Population
The inclusion criteria for the study were: 1) patients of age 18 years or above; 2) patients who had stable or unstable angina or acute recent myocardial infarction and; 3) patients who were undergoing coronary intervention with the study stent. The patients were excluded if they refused to give written informed consent or if they had any allergy to aspirin, clopidogrel, ticlopidine, heparin, cobalt chromium, sirolimus or polymers used in a study stent.
Description of the Study Stent
The Indolimus biodegradable polymer coated sirolimus-eluting coronary stent (Sahajanand Medical Technologies Pvt. Ltd., Surat, India) has a strut thickness of 60 μm and drug load of 1.4μg/mm2. It involves L605 cobalt chromium (Co-Cr) alloy as its stent platform. Initially, 70% of drug is released within 7 days and remaining drug is released over a period of 48 days [Table/Fig-1]. The drug is released within 7 weeks after the stent implantation from the polymeric layers coated onto the surface of the stent. The biodegradable polymeric film is a blend of different biodegradable polymers- poly L-lactide, 50/50 poly DL-lactide-co-glycolide and polyvinyl pyrrolidone, which undergoes hydrolysis. This process takes approximately 9 to 12 months after which all the polymer degrades naturally and excretes from body in the form of their metabolites.
In-vitro drug release from the Indolimus stent
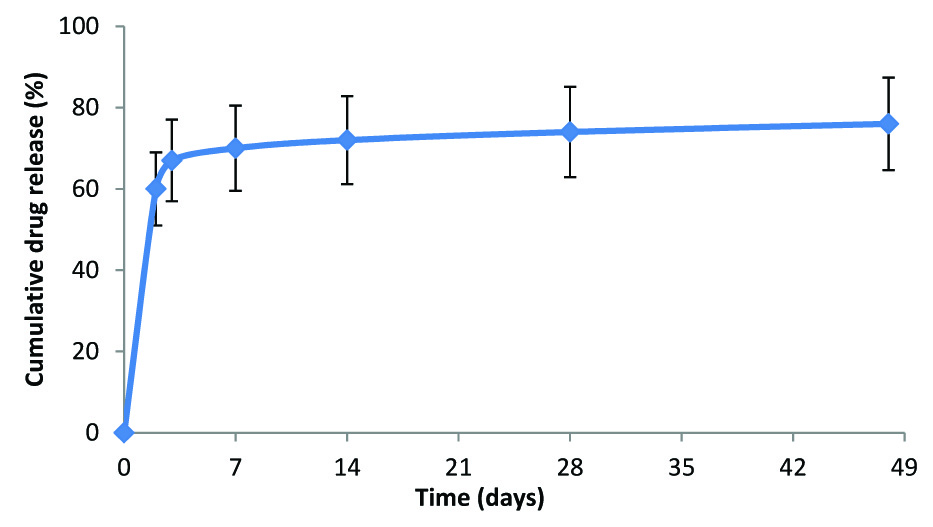
The average coating thickness of Indolimus stent is between 5 to 6 μm. The Indolimus stent is available in lengths of 8, 12, 16, 20, 24, 28, 32, 36 and 40 mm and available diameters were 2.5, 2.75, 3.0 and 3.5 mm.
Interventional Procedure and Adjunctive Medication
All patients received a loading dose of 300 mg of aspirin and clopidogrel (300 mg) or prasugrel (60 mg) or ticagrelor (90 gm). The procedural anticoagulation was achieved either with heparin or bivalirudin. However, the intra-procedural administration of glycoprotein IIb/IIIa-inhibitor was at the investigator’s discretion. The procedure was performed according to the standard treatment guidelines of each participating centre. All the patients received dual antiplatelet therapy (aspirin 75-300 mg/daily indefinitely and clopidogrel 75 mg/daily or prasugrel 10 mg/daily or ticagrelor 90 mg twice daily for at least 6 months) after the procedure.
Endpoints of the Study
The primary end-point of the study was major adverse cardiac events (MACE), which is a conglomeration of cardiac death, myocardial infarction (MI) (Q-wave and non-Q-wave), target lesion revascularization (TLR), target vessel revascularization (TVR) and stent thrombosis (ST). These end-points were observed at 30-days, 6-months and 9-months follow-up. The secondary endpoints will be measured at 12 and 24 months and yearly thereafter for five years.
Definition of Endpoints and Clinical Events
Procedural success was defined in terms of in-hospital MACE. MACE is composed of cardiac death, MI, TLR and TVR. Death can be cardiac or non-cardiac death. Any death due to undetermined cause was reported as cardiac death. Q-wave MI was considered when there was development of new Q-wave of more than 0.04 seconds in two or more adjoining leads along with increase in cardiac markers like Troponin I or T, creatine kinase or MB isoform. Non Q-wave MI was considered when there was more than three times elevation in creatinine kinase levels along with elevation in MB isoform and Troponin markers T or I without development of new Q-waves. Target lesion revascularization was considered when there was stenosis in treated segment (5mm proximal and 5mm distal edges) [12]. Target vessel revascularization was considered when there was stenosis in any segment of the treated vessel. Stent thrombosis (ST) was categorized according to Academic Research Consortium (ARC). It was considered acute when it occurred within 24 hours, sub acute when it occurred between 1 and 30 days and late when it occurred after 30 days. The ‘definite’ stent thrombosis was counted when it was detected angiographically. If the patient had a target vessel–related MI or died of a coronary event, it was “probable”, and “ possible” if any unexplained death occurred from 30 days after intracoronary stenting.
Follow-Up
Clinical follow-up, by telephonic conversation were scheduled at 30-days (± 7 days window period), 6-months (±15days window period) and 9-months (±30days window period). Follow-up data were collected pertaining to current clinical status, prior hospitalisation and occurrence of any of the aforementioned adverse events.
Statistical Analysis
Continuous variables are presented as mean ± standard deviation and categorical variables as counts and percentages. The event free survival curve was calculated according to the Kaplan-Meier method. All data were analysed using the Statistical Package for Social Sciences (SPSS; Chicago, IL, USA) program, version 15.
Results
Baseline Demographics and Lesion Characteristics
A total of 1008 patients were enrolled in the study. The basic demographic details of the patients are outlined in [Table/Fig-2]. The mean age was found to be 52.6 ± 11.0 years. Out of total patients, 740 (73.4%) were male, 372 (36.9%) had a history of diabetes mellitus and 408 (40.5%) had history of hypertension. A total of 1137 lesions were intervened successfully with 1242 stents (1.09 ± 0.30 per lesion). The average stent length and diameter were 27.42 ± 9.01 mm and 3.12 ± 0.36 mm respectively. According to ACC/AHA classification, there were 491 (43.2%) type B lesions, 604 (53.1%) type C lesions and 170 (16.9%) totally occluded lesions. The lesion and angiographic procedural details are outlined in [Table/Fig-3].
Baseline demographic characteristics
| Characteristics | Indolimus SESn = 1008 patients |
|---|
| Age (mean ± SD, yrs) | 52.6 ± 11.0 |
| Male, n (%) | 740 (73.4%) |
| Diabetes mellitus, n (%) | 372 (36.9%) |
| Hypertension, n (%) | 408 (40.5%) |
| Hypercholesterolemia, n (%) | 68 (6.7%) |
| Smoker, n (%) | 237 (23.5%) |
| Previous stroke, n (%) | 17 (1.7%) |
| Previous MI, n (%) | 115 (11.4%) |
| Primary PCI, n (%) | 166 (16.5%) |
SES-sirolimus-eluting stent, MI-myocardial infarction, PCI-percutaneous coronary intervention
Lesion and procedural characteristics
| Characteristics | Patients = 1008 / Lesions = 1137 |
|---|
| Lesion location |
| Left anterior descending, n (%) | 510 (44.9%) |
| Right coronary artery, n (%) | 386 (33.9%) |
| Left circumflex, n (%) | 239 (21.0%) |
| Left main, n (%) | 2 (0.20%) |
| ACC/AHA lesion classification |
| A, n (%) | 42 (3.7%) |
| B1, n (%) | 149 (13.1%) |
| B2, n (%) | 342 (30.1%) |
| C, n (%) | 604 (53.1%) |
| No. of diseased vessels |
| Single vessel disease, n (%) | 687 (68.2%) |
| Double vessel disease, n (%) | 286 (28.4%) |
| Triple vessel disease, n (%) | 35 (3.5%) |
| Total occlusion, n (%) | 170 (16.9%) |
| Total no. of stent | N = 1242 |
| No. of stents per patient, (mean ± SD, mm) | 1.23 ± 0.5 |
| Average stent length, (mean ± SD, mm) | 27.42 ± 9.0 |
| Average stent diameter, (mean ± SD, mm) | 3.12 ± 0.36 |
ACC/AHA- American College of Cardiology/ American Heart Association
Clinical Outcomes
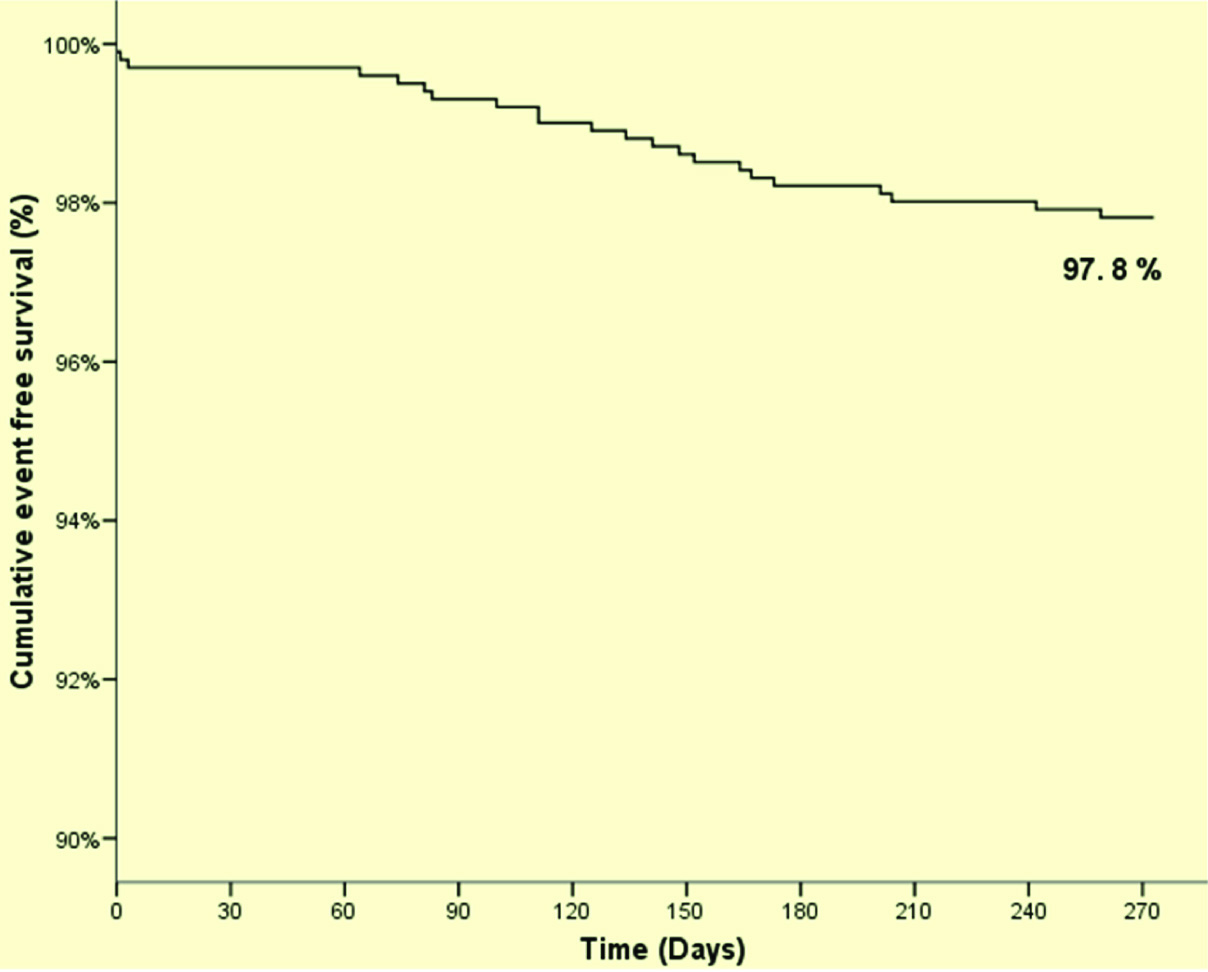
The incidence of MACE at 30-days, 6-months, and 9-months was found to be 3 (0.30%), 18 (1.80%) and 22 (2.20%) respectively. The cumulative event free survival by Kaplan Meier method was found to be 97.8%. The detailed clinical outcomes of the study are outlined in [Table/Fig-4,5]. Long term follow-up of the study would further confirm safety and efficacy.
Cumulative event-free survival curve at 9-month follow-up
Cumulative major adverse cardiac events at 30-days, 6-months and 9-months follow-up
| Events | 30-daysfollow-up(n=1008) | 6-monthsfollow-up(n=998) | 9-monthsfollow-up(n=998) |
|---|
| Death, n (%) | 3 (0.30%) | 4 (0.40%) | 4 (0.40%) |
| Cardiac death, n (%) | 3 (0.30%) | 4 (0.40%) | 4 (0.40%) |
| Non-cardiac death, n (%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Myocardial infarction, n (%) | 0 (0%) | 7 (0.70%) | 10 (1.0%) |
| Q-wave, n (%) | 0 (0%) | 3 (0.30%) | 4 (0.40%) |
| Non Q-wave, n (%) | 0 (0%) | 4 (0.40%) | 6 (0.60%) |
| TLR, n (%) | 0 (0%) | 5 (0.50%) | 6 (0.60%) |
| TVR, n (%) | 0 (0%) | 0 (0%) | 0 (0%) |
| ST, n (%) | 0 (0%) | 2 (0.20%) | 2 (0.20%) |
| Definite, n (%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Probable, n (%) | 0 (0%) | 2 (0.20%) | 2 (0.20%) |
| Total MACE | 3 (0.30%) | 18 (1.80%) | 22 (2.20%) |
TVR - target vessel revascularization; TLR - target lesion revascularization, ST - stent thrombosis
Discussion
The introduction of drug eluting stents has been a breakthrough discovery in the field of interventional cardiology. There was a steep decrease in the rates of restenosis and revascularization with DES as compared to BMS [6,13,14]. Metamorphically, Newton’s law of motion, ‘For every action there is an equal and opposite reaction’, applies here also. After initial enthusiasm, there have been questions regarding long term safety of DES due to its potential for increased risk of stent thrombosis. The persistent presence of durable polymer, even after elution of drug, instigates local inflammatory reaction and delayed arterial healing leading to late stent thrombosis. The continuous efforts of researchers to achieve better than the best, emerged as biodegradable polymer coated drug eluting stents.
Our results demonstrate that at the end of 6-months, the Indolimus SES was associated with ST rates as low as 0.2%. Amid the fireworks, there lies skepticism whether the decrease in thrombogenic potential is solely because of biodegradable polymer usage or not. A new concept on ST proposed by Kolandaivelu et al., explains using an ex-vivo model that strut thickness, geometry and optimal positioning, along with biodegradable polymer usage are critical factors in reducing thrombosis risk [15].
Indolimus, which is a biodegradable polymer coated sirolimus-eluting stent, has a strut thickness of 60 μm. This low-profile stent with cobalt-chromium stent platform has serpentine struts for homogenous stress distribution upon expansion. This unique design provides optimum radial strength and trackability. In vivo stent recoil assessment of one of the stent, similar to Indolimus in characteristics and design, revealed that acute stent recoil has higher radial strength compared to other available stents [16].
The Indolimus registry, which is a post-marketing surveillance registry of Indolimus SES, demonstrated favourable outcomes at 6-months follow-up [17]. The incidence of MACE was found to be 3.40%, which is a composite of 13 (2.45%) cases of cardiac death and 2 cases of (0.38%) myocardial infarction. Similar reproducible results are obtained in this multi-centre 9-months study. At 9-month, the occurrence of MACE was 2.20%, which is composite of 4 (0.40%) cases of cardiac death, 10 (0.90%) cases of myocardial infarction and 6 (0.50%) cases of TLR and 2 (0.20%) cases of ST.
In another registry, which aimed at evaluating safety and efficacy of biodegradable polymer coated SES, MACE was found to be 12 (2.1%) with TLR as low as 3 (0.5%) at 6-months follow-up [18]. The results of our study are quite comparable with this registry and delineate safety and efficacy of the Indolimus.
The current state-of-art in interventional cardiology suggests that any new emerging stents will require enormous efforts to prove its superiority (or even non-inferiority) and establish safety and efficacy. More complex lesions, larger population size and longer follow-ups have become mandatory [19]. The INDLOIMUS - G enrolling large cohorts of unselected patients undergoing PCI, involves patients at high risk and complex coronary lesions (36.9% DM, 16.5% primary PCI and 15% CTO).
Thus, it is evident that combination of stent geometry, thin strut, and biodegradable polymer usage has contributed to excellent clinical performance of the Indolimus SES. But it is also evident that expected potential of biodegradable polymer can emerge only after long-term follow-up, after complete absorption of biodegradable polymer. So, long-term follow-up study would further demonstrate prolonged safety and efficacy of the Indolimus SES.
Study Limitation
The main limitation of the study is that it was a single-arm, non-randomized study.
Conclusion
The use of Indolimus is associated with lower incidence of TLR, ST and consequent MACE. Thus, the INDOLIMUS - G Registry gives an idea about favourable safety, efficacy and clinical performance of the Indolimus in large cohorts of unselected patients with complex coronary artery lesions.