H’ Shaped Bilobate Placental Partition: A Rare Placenta Variation
Victor Okoliko Ukwenya1, Adam Moyosore Afodun2, Khadijah Kofo Quadri3, Olumide James Ashaolu4
1 Faculty, Department of Anatomy, College of Medicine, Ekiti State University, Ado-Ekiti, Nigeria.
2 Faculty, Department of Radiology, Ultrasound Unit, Crystal Specialist Hospital, Akowonjo-Egbeda, Lagos.
3 Faculty, Department of Physiology, College of Medicine, University of Lagos, Lagos, Nigeria.
4 Faculty, Department of Anatomy, Faculty of Basic Medical Sciences, Bowen University, Iwo, Nigeria.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Victor Okoliko Ukwenya, Faculty, Department of Anatomy, College of Medicine, Ekiti State University, Ado-Ekiti, Nigeria. E-mail : victorwyn@yahoo.com
Anomalies of placental growth with respect to its shape have been associated with adverse pregnancy outcomes. A very rare form of ‘H’ bilobate placental partition was observed in a 31-year-old woman presenting at 24 weeks of gestation. This observation was made during a routine obstetrics scan. Sonographic features showed that it was a non-fibroid partition. The placenta featured anterior and posterior portions separated by a large middle, vertical portion. The placenta was antero-posterior semi-circumferential in shape, measuring approximately 16.70 cm in length and 12.48 cm at shorter chorionic plate (minor axis). The middle, vertical part of the placenta partitioned the uterine cavity into two, creating the impression of two separate gestational sacs. The fetus was located in one of these. The patient was eventually delivered through caesarean section at gestational age (GA) of 36 weeks 6 days. The baby had a low birth weight of 1.70 kg. The early detection of this placental anomaly underlines the importance of ultrasonography in obstetrics.
Anomaly, Obstetrics, Ultrasonography
Case Report
A 31-year-old pregnant woman came to the Ultrasound Unit of Crystal Specialist Hospital, Egbeda, Lagos, Nigeria for routine obstetrics scan. Siemens Ultrasound machine (Sonoline 450 SL, made in Germany) with a 3.5 MHz probe was used to perform complete obstetric scan. Transabdominal scan revealed a distinct H-shaped placenta in a singleton pregnancy with breech presentation. Sonographic features showed that it was a non-fibroid partition. The placenta featured an anterior and posterior portions separated by a large middle, vertical part [Table/Fig-1,2]. Cord insertion was eccentric at 3 O’clock position. The placenta had two umbilical arteries and one umbilical vein and a coil index of 1 to 5.87cm. The middle, vertical portion of the placenta partitioned the uterine cavity into two, creating the impression of two separate gestational sacs. The fetus was located in one of these [Table/Fig-1,2].
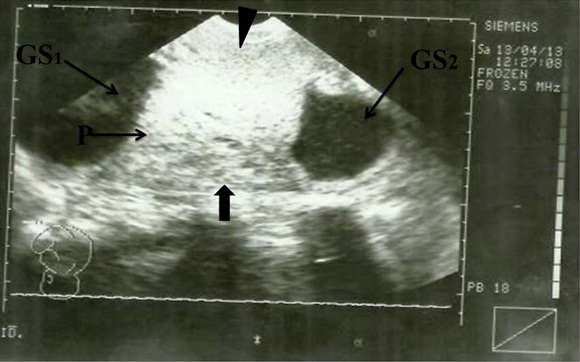
Sonogram showing H-Shaped Placenta. AC, Abdominal Circumference; P, Placenta (main); Arrowhead indicates anterior segment of placenta; Block Arrow indicates posterior segment of placenta. GS1, Gestational sac 1; GS2, Gestational sac 2
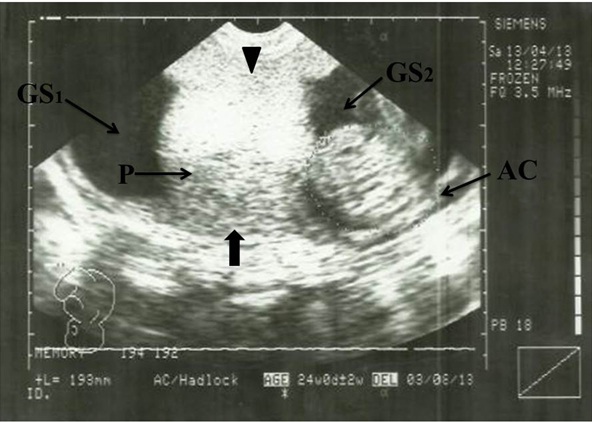
Sonogram showing H-shaped placenta. P, Placenta (main); Arrowhead indicates anterior segment of placenta; Block arrow indicates posterior segment of placenta; GS1, Gestational sac 1; GS2, Gestational sac 2
The placenta was antero-posterior semi-circumferential in shape, measuring approximately 16.70 cm in length and 12.48 cm at shorter chorionic plate (minor axis). The fetal abdominal circumference (AC) was 193 mm which corresponded to a GA of 24 weeks and the expected date of delivery (EDD) was 03/08/2013 [Table/Fig-1].
The patient had no history of diabetes mellitus, hypertension, anaemia or tuberculosis. There was no occurrence of bleeding or diminished twin /‘vanished twin’ in the first trimester. The patient was eventually delivered through caesarean section at GA of 36 weeks 6 days. The baby weighed 1.70 kg at birth with no apparent congenital malformation. Following delivery the placental weight (untrimmed) was 601.82 gm with presence of two thrombi, seven cotyledons and three infarcts. Disc outline showed no evidence of placental membranacea, percreta, increta or acreta. Disc thickness was 2.87 cm thick having convex-shape, with normal umbilical cord coil index.
Discussion
The placenta is derived from two separate individuals, the mother and the fetus [1]. The human placenta consists of a villous tree which is bathed in maternal blood. The villous tree connects the fetal and maternal surfaces of the placenta together, thereby facilitating the exchange of oxygen and nutrients between maternal and fetal blood [2]. An irregular maternal utero-placental environment affects macroscopic placental structure causing a change in its shape [3]. Studies have shown that irregular placental shapes are associated with deformation of the placental vascular network and diminished placental efficiency, with potential for negative perinatal outcomes [4].
Various kinds of placental shape abnormality have been reported in literature. They include bi-discoidal, lobed, placenta membranacea, placenta succenturiata, fenestrated placenta, circumvallate and circummarginate placentae [5].
We reported a rare case of ‘H’ bilobate placental partition in which the fetus suffered the risk of oligohydramnios and insufficient space due to the partitioning of the uterine cavity. This underlying stress necessitated the delivery of the fetus through caesarean section. At birth, it was found to be of low birth weight, weighing 1.70 kg. Fetal growth disturbances like intrauterine growth restriction (IUGR) and small gestational age (SGA) are perinatal complications associated with maternal conditions such as pre-gestational and gestational diabetes [6], anaemia or tuberculosis [7]. However, in this case study, the mother had no such history. However, there is proven association between placenta location and intrauterine growth restriction (IUGR). Lateral placentation has four times the number of IUGR pregnancies compared with anterior or posterior placentation [8]. Other studies have also linked placenta praevia with intra-uterine growth retardation [9,10].
The low birth weight associated with the H-shaped placenta in this case might be related to the abnormal placental structure altering uteroplacental and fetal-placental circulations, resulting in reduced maternal uteroplacental blood flow, caused by insufficient or incomplete trophoblastic invasion of the spiral arteries in the placental bed. This supports assertions that placental structure and function determine the growth and development of the fetus [4]. Improper development and functioning of the placenta affects the fetus, causing prematurity [11] and fetal growth and neuro developmental abnormalities [12].
The H-shaped placental partition described in this case has not been previously portrayed in literature; it is indeed a rare form of placenta. The mechanism of its formation remains a point of scientific interest. It could have been derived by the merging of a succenturiate lobe with a main implantation. It might have also been derived by the merging of the anterior and posterior segments of a bilobed placentation, in which case we might describe it as a rare form of bilobate placenta.
Conclusion
The early detection of this placental anomaly by ultrasonography (USG) underlines the importance of USG in obstetrics, especially in the environment in which this case was noted, where USG is not considered as ‘mandatory’ due to ignorance and superstitious beliefs. It is important to note that incidents of ‘structural-placental-incapability’, structural deviation or underlying diseased conditions can affect post-natal development.
Literature is scanty on congenital placental anomalies and anatomic variation. Weight studies and their roles in the possible disruption of nutrient/oxygen transfer to the fetus in uterus may have parallels to small for gestational age (SGA) & IUGR. Post-natal histopathology of syncytiotrophoblast (syncytium) is recommended for future studies to provide a mirror into syndromes occurring in pregnancy. Four (4) dimensional i.e. (3D in real-time) USG reconstruction is also recommended for future encounter.
[1]. Manae P, Silotry N, Mukherjee A, Bhandari K, Acharya S, A comparative study of placenta in normal and pregnancy induced hypertensionInternational Journal of Scientific Research 2014 3(6):286-88. [Google Scholar]
[2]. Jaffe R, Jauniaux E, Hustin J, Maternal circulation in the first-trimester human placenta–myth or reality?Am J Obstet Gynecol 1997 176(3):695-705. [Google Scholar]
[3]. Biswas S, Ghosh SK, Gross morphological changes of placentas associated with intrauterine growth restriction of fetuses: a case control studyEarly Hum Dev 2008 84(6):357-62. [Google Scholar]
[4]. Salafia CM, Yampolsky M, Misra DP, Shlakhter O, Haas D, Eucker B, Placental surface shape, function, and effects of maternal and fetal vascular pathologyPlacenta 2010 31(11):958-62. [Google Scholar]
[5]. Sudha R, Sivakumar V, Christilda FJ, Study of shape of placenta and its relation to placental weight in normal and complicated pregnanciesNational Journal of Basic Medical Sciences 2012 2(4):307-11. [Google Scholar]
[6]. Ornoy A, Prenatal origin of obesity and their complications: gestational diabetes, maternal overweight and the paradoxical effects of fetal growth restriction and macrosomiaReprod Toxicol 2011 32(2):205-12. [Google Scholar]
[7]. Krishna U, Bhalerao S, Placental insufficiency and fetal growth restrictionJ Obstet Gynaecol India 2011 61(5):505-11. [Google Scholar]
[8]. Kalanithi LE, Illuzzi JL, Nossov VB, Frisbaek Y, Abdel-Razeq S, Copel JA, Intrauterine growth restriction and placental locationUltrasound Med 2007 26(11):1481-89. [Google Scholar]
[9]. Ananth CV, Demissie K, Smulian JC, Vintzileos AM, Relationship among placenta previa, fetal growth restriction, and preterm delivery: a population-based studyObstet Gynecol 2001 98(2):299-306. [Google Scholar]
[10]. Dommisse J, Placenta praevia and intra-uterine growth retardationS Afr Med J 1985 67(8):291-92. [Google Scholar]
[11]. Rees S, Inder T, Fetal and neonatal origins of altered brain developmentEarly Hum Dev 2005 81(9):753e61 [Google Scholar]
[12]. Guttmacher AE, Maddox YT, Spong CY, The human placenta project: Placental structure, development, and function in realtimePlacenta 2014 35(5):303-04. [Google Scholar]