Cross contamination is a problem confronting all members of dental profession. Prosthodontic patients are generally at a high-risk in their potential to transmit infectious diseases as well as to acquire them. Although care is required for every step of fabrication of prosthesis but impressions are considered to be one of the largest source for transfer of the potentially infectious material [1,2].
The dental impression is a potential source of infection in prosthodontic practice. Saliva, blood and plaque-contaminated impressions may harbour pathogenic organisms and may pass from patients to dental personnel who handle the impression or subsequent casts. Various non elastic and elastic impression materials are used for making impressions. Depending upon the hydrophilic nature of the materials, the presence or absence of surfactant and their tolerance to immersion in water or other fluids are the key elements in understanding the disinfection protocols for impression materials. For instance, polyether impression material is hydrophilic in nature, thus absorbs water and other liquids resulting in dimensional changes. Also, the loss of surfactants affects the wettability of the impression. However, alginates are sensitive to both wet and dry environments [3].
Various methods used for disinfecting impressions are technique sensitive and time consuming. Some disinfectant solutions may cause significant changes in impression, particularly with over exposure. These solutions may produce irritating vapours, depending on the disinfectant used. The use of UV rays can be a good alternative choice for disinfection because UV Chambers are available in most of the dental clinics and are used to store sterilized dental instruments to avoid recontamination from dental operatory.
Ultraviolet rays have long been recognized as an effective method for killing microbes without requiring chemicals or heat. When microorganisms are exposed to UV rays at a particular wavelength (200-280 nm), their reproduction capability is destroyed and inactivation occurs at a faster rate, so that they no longer pose threat to humans [4]. So, this study was conducted to evaluate the efficacy of U.V rays to disinfect various impression materials at different time intervals was determined and was compared with 2% glutaraldehyde.
Materials and Methods
Materials
Alginate (Irreversible hydrocolloid impression material, Plastalgin, Septodont, France).
Addition silicone impression material (Putty- Light body, 3M ESPE, Bangalore, India).
Polyether impression material (Medium bodied, Impregnum 3M ESPE Monophase, Bangalore, India).
2% Glutaraldehyde solution (CIDEX Johnson & Johnson company,
Ultraviolet rays chamber (S.K Dent, New Delhi, India).
Incubator (Obramax, O.P and Bros Scientific Works Delhi, India).
Digital Colony counter (Tanco, New Delhi, India).
Culture plates (Borosil Glass work Limited, New Delhi, India).
Ringers Solution (Himedia Laboratory, Mumbai, India).
Culture media (Himedia Laboratory, Mumbai, India).
Sterile punch cutter.
Methods
The impressions were made from 30 dentulous subjects with age ranging from 18 to 28 years. One impression was made for each patient. Patients with stains, calculus, caries and missing teeth were excluded. Ten impressions were made for each impression material i.e. alginate, addition silicone and polyether impression material [Table/Fig-1–3] and thus a total of 30 impression were made for the three materials. Three impressions were made in a day with an incubation period of 72 hours respective to study protocol. Six punch samples were taken from each impression [Table/Fig-4,5]. Thus a total of 180 punch samples were taken from various impression materials i.e. alginate, addition silicone and polyether impression material. Out of 6 punch sample, one was kept as control, second was disinfected by immersing in freshly prepared 2% glutaraldehyde solution for 10 minutes and remaining four were exposed to UV rays for 3 minutes, 6 minutes, 10 minutes and 15 minutes using dental UV chamber.
(1) Alginate Impression. (2) Addition Silicone Impression. (3) Polyether Impression. (4) Use of sterile punch cutter for cutting samples. (5) Punch Sample. (6) UV Chamber. (7) Samples immersed in Ringers Solution after disinfection by various methods. (8) Turbidity observed over peptone water. (9) Pour Plate technique. (10) Digital colony counter. (11) Counting of colonies over digital colony counter. (12) Observation of colonies over culture plates – [i] Control group showing maximum colonies. [ii] 3 minutes of exposure to UV Rays. [iii] 6 minutes of exposure to UV Rays. [iv] 10 minutes of exposure to UV Rays. [v] 15 minutes of exposure to UV Rays. [vi] After immersion in 2% Glutaraldehyde solution for 10 minutes.
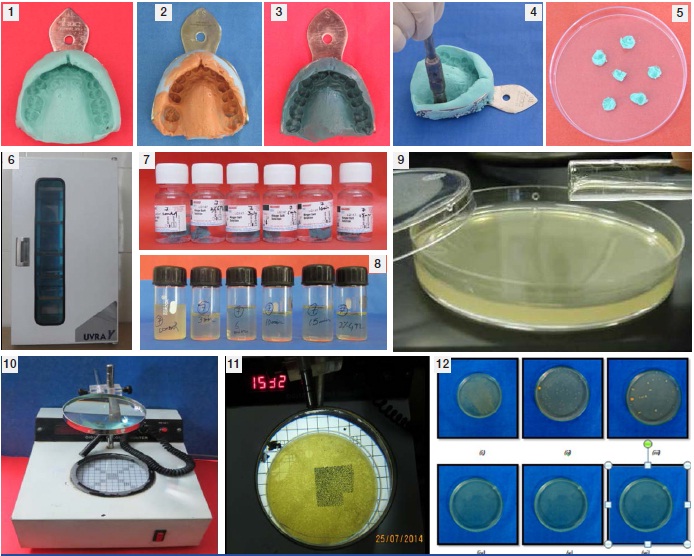
Sample 1 was served as control group i.e. at room temperature in sterile glass container. Sample 2 was disinfected by immersing in freshly prepared 2% glutaraldehyde and then washed with sterile distilled water. Sample 3, 4, 5 and 6 were disinfected by exposure of UV light for different time intervals i.e. 3minutes, 6 minutes, 10 minutes and 15 minutes respectively by keeping them in an ultra-violet chamber with wavelength of 254 nm [Table/Fig-6]. Different disinfection procedures were performed at room temperature and the samples prepared were again rinsed with sterile distilled water for 15 seconds to remove any traces of the disinfectant from the impression surface.
After disinfection each punch sample was immersed in a tube containing Ringer’s solution (10 ml) and then incubated for 2 hours at 37°C so that the micro-organisms get suspended into the solution [Table/Fig-7]. After incubation, 1 ml of Ringers solution containing eluted microorganisms from each tube was inoculated in a tube containing 9 ml of broth and was incubated again at 37oc for 24 hours for the growth of micro organisms. After 24 hours, tubes were observed for the turbidity for growth [Table/Fig-8].
Culture broth containing growth of each sample was inoculated over the solid agar media with the help of pour plate method for the observation and counting of colonies [Table/Fig-9]. After inoculation, media plates were incubated at 37°C for 24 hours. The media plates were then observed for the colonies after 24 and 48 hours for the confirmation of the results. Finally Colony forming units (CFU) were counted using digital colony counter and results were compared with control group and subjected to statistical analysis [Table/Fig-10–12].
Results
The results of the foregoing study are tabulated from [Table/Fig-13,14 and 15] and plotted in. [Table/Fig-13] shows one-way ANOVA test showing mean colony count over alginate impression by various disinfection techniques. The colonies counted after employing the various techniques such as 2% glutaraldehyde, 3 minutes, 6 minutes, 10 minutes and 15 minutes UV exposure along with the control group depicted statistically highly significant differences. For control group, the mean value counted was 11797.40±5989.73 (mean ± SD). For 3 minutes of UV exposure mean value counted was 6780±5545.60 (mean ± SD) and for 6 minutes of UV exposure the mean value calculated was 4546.52±1918.90 (mean ± SD).
One-way ANOVA Test showing mean colonies count over Alginate Impression by various disinfection techniques.
| Alginate Impression Material | Colonies Counted |
|---|
| Number | Mean | S.D. | p-value |
|---|
| Control | 10 | 11797.40 | 5989.73 | 0.001* |
| 2% Glutaraldehyde | 10 | 0.00 | 0.00 |
| 3 Minutes UV Exposure | 10 | 6780.01 | 5545.60 |
| 6 Minutes UV Exposure | 10 | 4546.52 | 1918.90 |
| 10 Minutes UV Exposure | 10 | 0.00 | 0.00 |
| 15 Minutes UV Exposure | 10 | 0.00 | 0.00 |
| Total | 60 | 3210.32 | 5880.77 |
One-way ANOVA Test showing mean colonies count over Addition silicone impression by various disinfection technique.
| Addition Silicone Impression Material | Number | Mean | S.D. | F value | p-value |
|---|
| Control | 10 | 7095.40 | 4268.83 | 20.393 | 0.001* |
| 2% Glutaraldehyde | 10 | 0.00 | 0.00 |
| 3 Minutes UV Exposure | 10 | 1734.76 | 1001.20 |
| 6 Minutes UV Exposure | 10 | 1388.38 | 449.00 |
| 10 Minutes UV Exposure | 10 | 19.92 | 6.30 |
| 15 Minutes UV Exposure | 10 | 0.00 | 0.00 |
One-way ANOVA Test showing mean colonies count over Polyether impression by various disinfection technique.
| PolyetherImpression Material | Colonies Counted |
|---|
| Number | Mean | S.D. | F-value | p-value |
|---|
| Control | 10 | 2168.92 | 1676.00 | 5.971 | 0.001 * |
| 2% Glutaraldehyde | 10 | 0.00 | 0.00 |
| 3 Minutes UV Exposure | 10 | 0.00 | 0.00 |
| 6 Minutes UV Exposure | 10 | 0.00 | 0.00 |
| 10 Minutes UV Exposure | 10 | 0.00 | 0.00 |
| 15 Minutes UV Exposure | 10 | 0.00 | 0.00 |
[Table/Fig-14] shows one-way ANOVA test showing mean colony count over addition silicone impression material by various disinfection techniques. The colonies counted for control group was found to be 7095.40 ± 4268.83 (mean ± SD), CFUs for 3 minutes of UV rays was 1734.76 ± 1001.20 (mean ± SD), for the 6 minutes of exposure to UV rays number of colonies were found to be 1388.38 and for 10 minutes mean count of colonies was 19.92 ± 6.30 (mean ± SD) depicted statistically highly significant differences.
[Table/Fig-15] shows one-way ANOVA test showing mean colony count over polyether impression material by various disinfection techniques. The colonies count for control group was 2168.92±1676 (mean ± SD) whereas no colonies were found on 3 minutes, 6 minutes, 10 minutes and 15 minutes of UV exposure that depicted statistically highly significant differences.
Discussion
Effective infection control is mandatory to reduce the potential of disease transmission during dental treatment. A large number of disinfectants are however available in market but it is not necessary that all disinfectants are compatible with every impression material. They may affect the surface details, dimensional stability and surface roughness of the impression thus affecting the accuracy of impression. Each of these disinfectants carries their own set of advantages as well as disadvantages which have been proved in various studies. For example, some researches indicate 2% glutaraldehyde as an ideal solution for disinfection, however other researchers have shown that the high toxicity of glutaraldehyde makes it unsuitable for daily clinical use [5].
Various factors that affect the effectiveness of Ultra-Violet light are time, intensity, humidity and direct access to the organism. Since dental prostheses do not get exposed from all areas, it is necessary that UV light must be reflected from many directions. While exposing an item frequent orientation increases the chances of killing micro organisms [6].
UV light of 200-280 nm wavelengths is lethal to bacteria, bacterial spores, viruses, mold, mold spores, yeast, and algae. Since the penetrating power of UV light is low, so it is not readily absorbed by organic materials. Before UV light disinfection, cleaning of visibly soiled surfaces is necessary [7]. While using dental UV chamber the wavelength used is 254 nm which is quite effective for disinfecting impression. Also the changes in the surface details as well as the dimensional accuracy of the impression are affected to a varying degree by these disinfectants.
This current study was conducted to evaluate the efficacy of UV Rays using Clinical UV Chamber which was used for storing sterilized instruments for disinfecting various impression materials at different time intervals and comparing it with 2% glutaraldehyde solution immersion for 10 minutes. Maximum numbers of colonies were observed in alginate impression materials followed by addition silicone impression material and least in polyether impression material. These findings were in agreement with the findings of Juvenicus J and Aljabrah et al., [8,9]. No researchers had proved otherwise. The micro-organisms transferred in alginate impressions were almost three to five times, the number of organisms that were transmitted in case of elastomeric impressions under the same conditions [10,11] as quoted by Jennings KJ, Samaranayake LP [12]. Powell also revealed the same result [13]. While other study revealed that Irreversible hydrocolloid had greater potential of retaining microorganisms, it retains bacteria 2 to 3 times higher than elastomers. Moreover, microorganisms were more persistent in alginate impression and this makes the process of disinfection difficult [4].
One-way ANOVA test was used for comparing the effect of freshly prepared 2% glutaraldehyde on all the three impression materials and it was found that there was 100% removal of microorganism. These results were consistent with the results of Johnson GH [14]. Many studies have reported the incompatibility of irreversible hydrocolloid with disinfectant solutions when immersed for more than 10 minutes that may cause dimensional changes. However, Rideouta K et al., & other researchers have shown that the high toxicity of Glutaraldehyde makes it unsuitable for daily clinical use [15].
The present study involves the use of UV rays from dental UV chamber which is used to store sterilized dental instruments and thus eliminates the possibility of surface deterioration as it does not involve immersion/spraying of the impression with disinfectant.
Conclusion
Alginate impressions showed significantly higher level of microbial growth than addition silicone with polyether showing the least growth. Equal amount of disinfection was however achieved with immersion in 2% glutaraldehyde for 10 minutes and on exposure to UV rays for 10 minutes.
For alginate impression material, disinfection was achieved on exposure to UV rays for a period of 10 minutes while in case of addition silicone impression material, no microbial growth remained on exposure to UV radiation for a time intervals of 10 minutes. Complete disinfection was attained on exposure for a period of 3 minutes to UV radiation in case of Polyether Impression Material.
So we can conclude that the dental UV Chamber being used for storage of sterilized dental instruments may also be used to disinfect dental impressions successfully.