This is the report of lower respiratory tract infection with Pasteurella canis in a chronic obstructive pulmonary disease (COPD) patient with history of casual exposure to cats. Pasteurella species are part of the oral and gastrointestinal flora in the canine animals. These organisms are usually implicated in wound infection following animal bites, but can also be associated with a variety of infections including respiratory tract infections.
Canine animals, Doxycycline, Vitek 2 system
Case Report
A 70-year-old male, hotel employee by occupation, known case of Chronic obstructive pulmonary disease (COPD) and ischaemic heart disease (IHD) presented to our hospital with a history of cough with purulent expectoration, low grade fever and worsening breathlessness of seven days duration. Patient had history of recurrent exacerbations of COPD caused by Pseudomonas spp. six months back. Patient was an active smoker and gave a history of casual exposure to domestic cats.
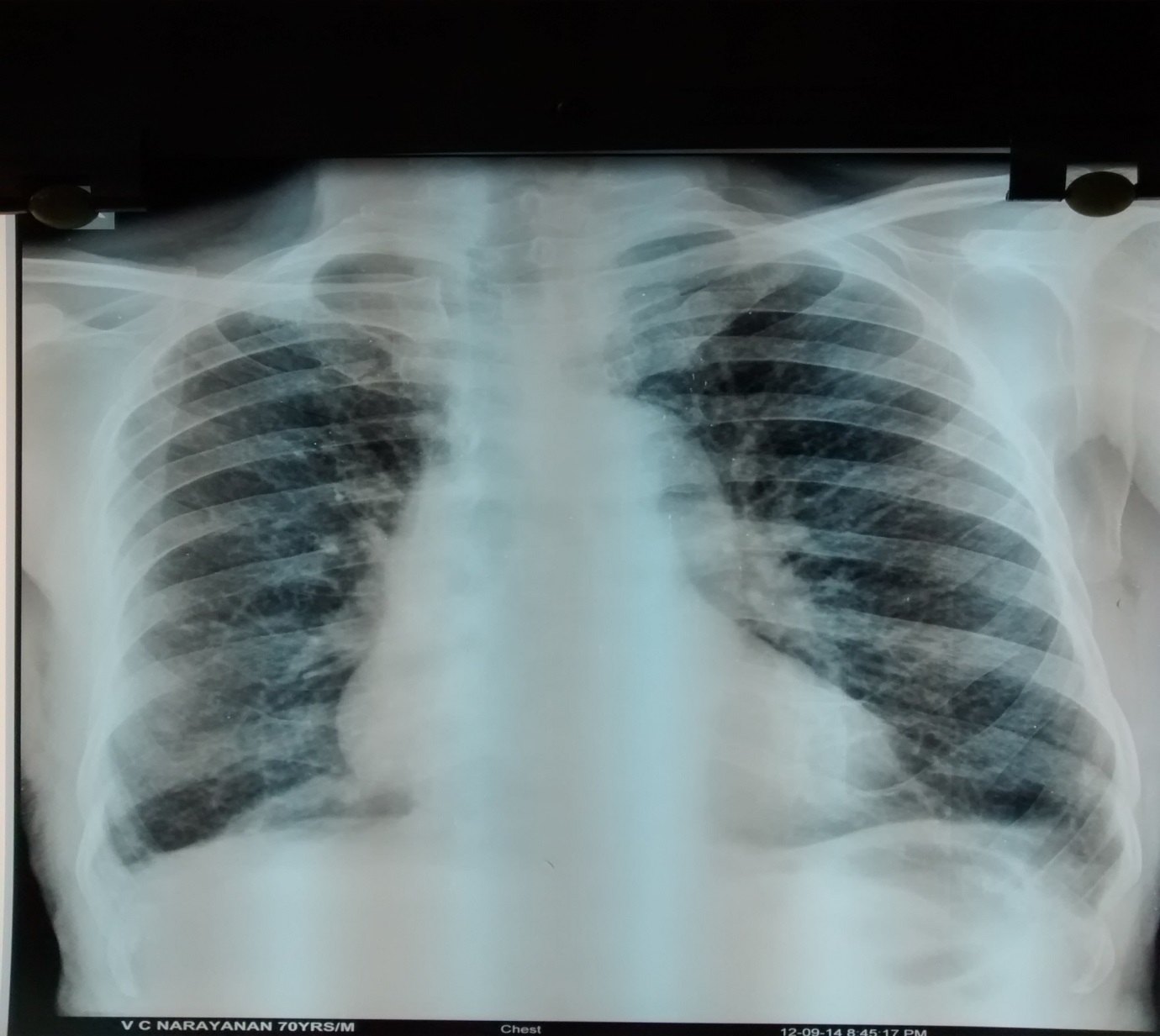
On examination, patient was conscious, afebrile, tachypneic (respiratory rate of 22/minute), mildly hypoxic (oxygen saturation on room air of 88% by pulse oximetry) and haemodynamically stable. Respiratory system examination revealed a barrel shaped chest and bilaterally diminished breath sounds with diffused polyphonic wheeze on auscultation. Routine blood investigations like haemogram, ESR, fasting blood glucose, renal function, serum electrolytes and liver function were all within normal limits. Arterial blood gas analysis was suggestive of mild Type I respiratory failure (pH= 7.36, PaCO2 = 36 mmHg, PaO2= 59.6 mmHg, PaHCO3= 21 mmHg). Chest radiograph showed changes of hyperinflation, unfolding of aorta and no evidence of lung parenchymal abnormalities [Table/Fig-1]. Spirometry was suggestive of severe obstructive impairment with no significant bronchodilator reversibility. Sputum was sent for gram stain, bacteriological culture and sensitivity testing. Patient was treated with low flow oxygen, ceftriaxone 1 gram intravenously BID, hydrocortisone and salbutamol + ipratropium nebulisations. Response to initial therapy at the end of 48 hours was poor.
Chest radiograph PA view showing hyper-inflated lung fields and an unfolded aorta
Gram stain smear of the sputum revealed numerous polymorphonuclear leucocytes with gram negative coccobacilli and it was decided to wait for the culture report before modifying the empiric antibiotic.
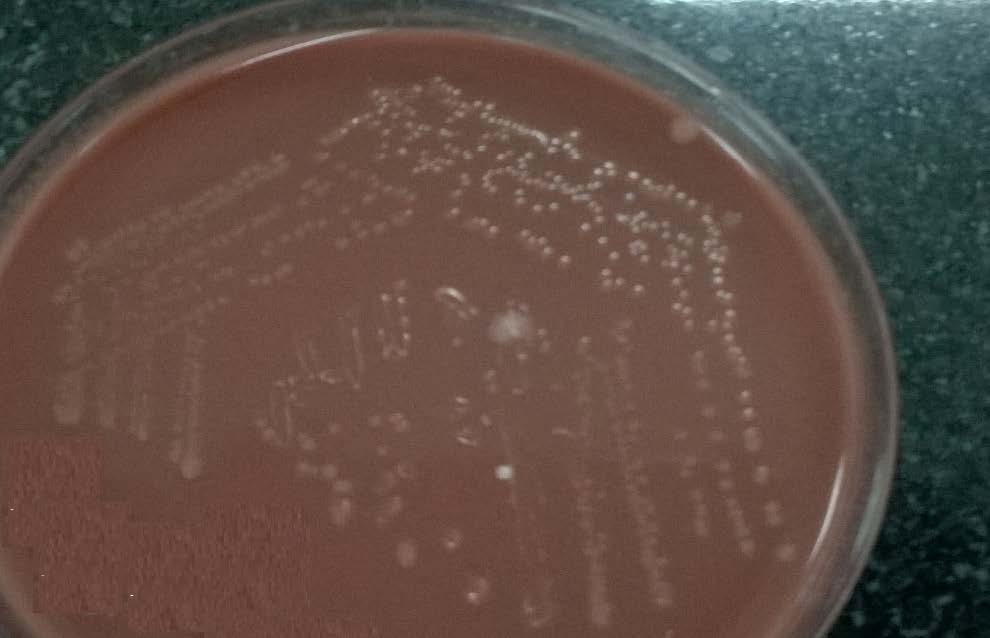
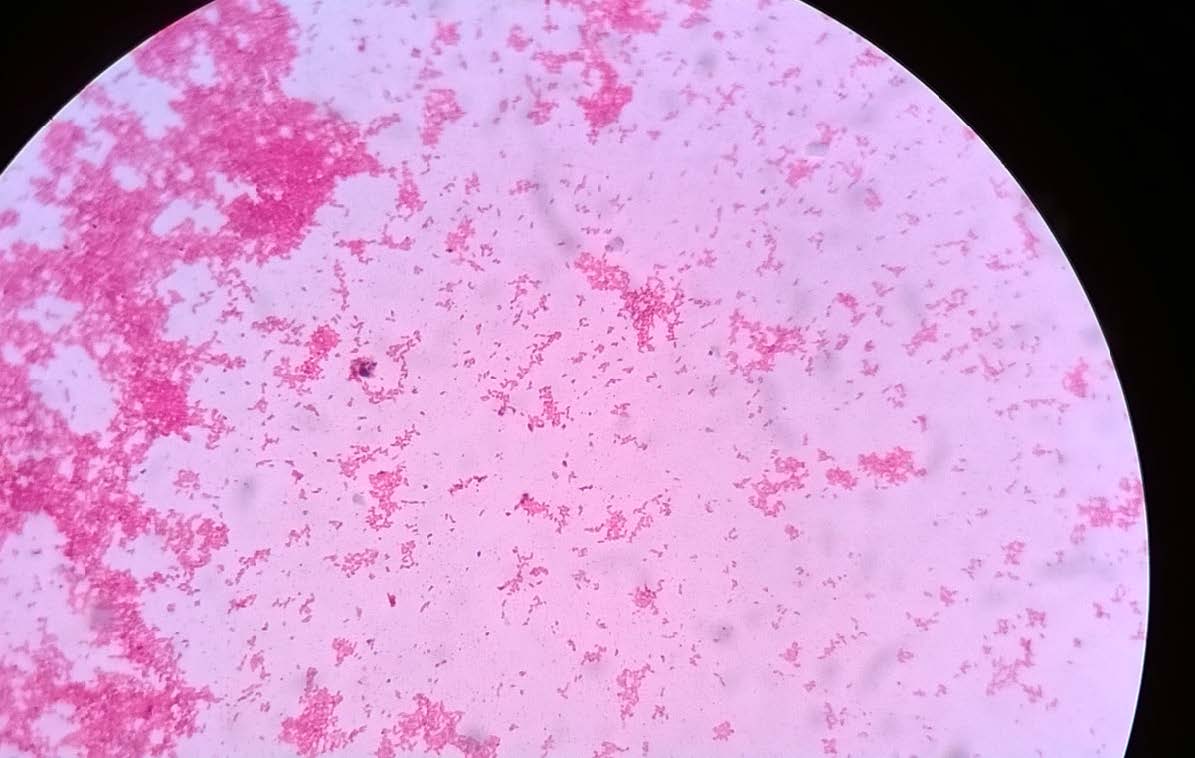
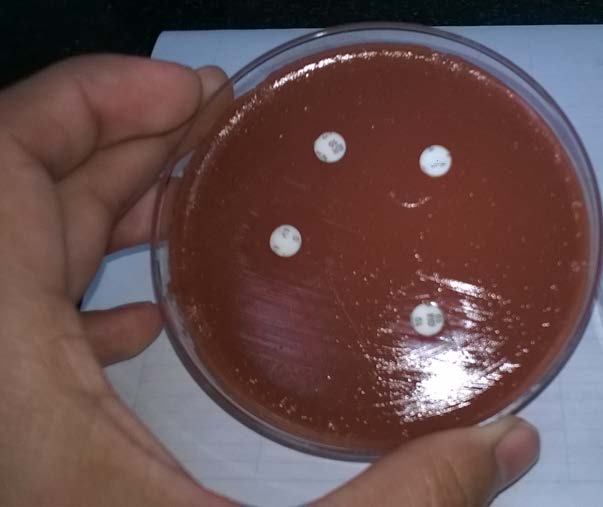
The sample was cultured on blood agar, chocolate agar and MacConkey’s agar plates and incubated at 37oC for 24 hours. Blood agar plates showed non haemolytic small dew drop colonies and chocolate agar plates showed small grey coloured colonies and the smear from the colonies showed the presence of gram negative coccobacilli [Table/Fig-2,3]. There was no growth on MacConkey’s agar plate. The isolate was catalase and oxidase test positive. The isolate was further identified as Pasteurella canis by Vitek 2 system (Bio-Mérieux, Co., Ltd.). Antibiotic susceptibility testing was done by modified Kirby-Bauer disk diffusion technique. The organism was sensitive to ciprofloxacin, amoxicillin-clavulanic acid, penicillin, gentamicin, clindamycin, levofloxacin, erythromycin, doxycycline and trimethoprim-sulfamethoxazole ([Table/Fig-4], interpretation with Haemophilus influenzae standards).
Culture on Chocolate agar plate showing smooth grey colonies of P.canis
Gram smear from the growth showing gram negative cocco bacilli
Antibiotic Susceptibility testing: plate showing zone of inhibition to Levofloxacin, Erythromycin, Gentamycin, Tetracycline
Taking into consideration the antibiotic sensitivity, parenteral ceftriaxone was stopped and replaced by oral doxycycline along with other supportive care. The patient showed gradual improvement and was discharged on oral doxycycline for two weeks along with inhaled bronchodilators. On follow up at two weeks, he was asymptomatic and a repeat sputum culture revealed no significant bacterial growth.
Discussion
Pasteurella canis is a gram-negative, non-motile coccobacillus or short rods belonging to the Pasteurellaceae family [1]. First referred to as “Micrococcus gallicidus”, the generic name was redesignated as “Pasteurella” in 1887 by Trevisan to commemorate the work of Pasteur on these bacteria. Like most species of Pasteurella, P. canis is oxidase and catalase test positive. It includes two biotypes: biovar 1 is originated from canine, whereas biovar 2 is originated from bovine animals. The two biotypes are distinguishable by the indole test: biovar 1 is indole positive whereas biovar 2 is indole negative [1].
P. canis are a part of the normal oropharyngeal flora of many animals including healthy dogs and cats. In humans, they are known to cause zoonotic infections. Human pasteurellosis most often results in skin or soft tissue infections after an animal bite. P. multocida is most commonly isolated in human infections but there have been reports of other species such as P. canis and P. dogmatis being involved [2]. P. canis is usually transmitted to human through animal bites, licks. Dog bites are most commonly implicated followed by cat bites. Exceptionally, some patients develop infections after other animal exposure and in some infection may occur even in the absence of an animal contact [3].
After soft tissue and wound infections, the respiratory tract is the second most common site for Pasteurella infection. Most patients with pulmonary infection due to Pasteurella are elderly with other pre-existing chronic lung diseases like COPD, bronchiectasis, or malignancy. The list of ‘pulmonary pasteurellosis’ includes tracheobronchitis, pneumonia, lung abscess and empyema [4]. P.canis causing bacteremia, peritoneal dialysis-related peritonitis, ocular infections including conjunctivitis outbreaks, osteomyelitis, cutaneous abscess and septic arthritis in the immunocompromised patients has been reported in the literature as well [5–9].
Pasteurella spp. is known to be susceptible to Penicillin G, amoxicillin-clavulanate, piperacillin, fluoroquinolones (levofloxacin, moxifloxacin), newer generation cephalosporins (ceftriaxone, cefixime, cefpodoxime), doxycycline and carbapenems. Treatment failures have been reported with the use of oral macrolides (e.g. erythromycin), oxacillin, dicloxacillin, first generation cephalosporins and clindamycin which should therefore be avoided [10].
Review of literature did not reveal any previous reports of P.canis being implicated as a co-pathogen in COPD exacerbations, although the organism itself finds mention as a causative agent in a multitude of other system disease usually against a background of intimate animal contact or trauma. In our patient, since there was only a casual contact with cats and no history of a scratch or a bite from the animal, we assume that he would have been exposed to secretions of his pet animal through inhalation of contaminated aerosol. The isolation of P. canis in the sputum of an elderly patient admitted with a COPD exacerbation and the fact that he had only an insignificant history of feline contact prompted us to report this case.
Kim et al., have also reported a case of respiratory tract infection caused by P. canis in a COPD patient (poodle owner). This bacteria is found in the oral secretions of canine animals and it can colonize and infect the respiratory tract in patients with lung disease. The patient was started on doxycycline and the symptoms improved. The presentation of this case is similar to our case [11].
T Akahane et al., have reported dual infection with Pasteurella dagmatis and P.canis in dog bite wound infection in a 25-year-old female [12]. The other infections caused by Pasteurella species reported in literature include cellulitis, subcutaneous abscesses following dog and cat bite, endocarditis following a cat-bite, vertebral osteomyelitis, spondylodiscitis in a diabetic patient [13,14]. Moreover, first case of association of P.canis, with bacteremia in a cirrhotic patient with open leg was reported by Albert et al., [15].
However to the best of our knowledge, this is the first case of exacerbation of COPD with Pasteurella species co-infection to be reported from this region.
Conclusion
Obtaining a detailed history of animal exposure in COPD patients is of paramount importance for the diagnosis of respiratory tract infection caused by Pasteurella spp. Elderly patients with COPD need to avoid close contact with pet animals as this could be a potential risk factor for pneumonia caused by P. canis.
[1]. Mutters R, IHM P, Pohl S, Frederiksen W, Mannheim W, Reclassification of the Genus Pasteurella Trevisan 1887 on the Basis of deoxyribonucleic acid homology, with proposals for the new species Pasteurella dagmatis, Pasteurella canis, Pasteurella stomatis, Pasteurella anatis, and Pasteurella langaaInt J Syst Bacteriol 1985 35(3):309-22. [Google Scholar]
[2]. Weber DJ, Wolfson JS, Swartz MN, Hooper DC, Pasteurella multocida infection. Report of 34 cases and review of the literatureMedicine 1984 63:133-53. [Google Scholar]
[3]. Rashid NK, Zam Z, MdNoor S, Siti-Raihan I, Azhany Y, Pasteurella canis isolation following penetrating eye injury: a case reportCase Rep Ophthalmol Med 2012 2012:362-69. [Google Scholar]
[4]. Klein NC, Cunha BA, Pasteurella multocida pneumoniaSemin Respir Infect 1997 12(1):54-56. [Google Scholar]
[5]. Hara H, Ochiai T, Morishima T, Arashima Y, Kumasaka K, Kawano KY, Pasteurella canis osteomyelitis and cutaneous abscess after a domestic dog biteJ Am Acad Dermatol 2002 46(5):S151-52. [Google Scholar]
[6]. Yefet E, Abozaid S, Nasser W, Peretz A, Zarfin Y, Unusual infection-Pasteurella canis bacteremia in a child after exposure to rabbit secretionsHarefuah 2011 150(1):13-5. [Google Scholar]
[7]. Hazelton BJ, Axt MW, Jones CA, Pasteurella canis osteoarticular infections in childhood: review of bone and joint Infections due to Pasteurella Species over 10 Years at a tertiary pediatric Hospital and in the literatureJ Pediatr Orthop 2013 33(3):e34-38. [Google Scholar]
[8]. Balikoglu-Yilmaz M, Yilmaz T, Esen AB, Engin KN, Taskapili M, Pasteurella canis and Granulicatella adiacens conjunctivitis outbreak resistant to empirical treatment in a child welfare agencyJ Pediatr Ophthalmol Strabismus 2012 49(5):314-19. [Google Scholar]
[9]. Castellano I, Marín JP, Gallego S, Mora M, Rangel G, Suarez MA, Pasteurella canis peritonitis in a peritoneal dialysis patientPerit Dial Int 2011 31(4):503-04. [Google Scholar]
[10]. Kaftandzieva A, Peneva M, Petrovska B, Cekovska Z, Pasteurella Canis as a cause of soft-tissue infection after dog bite: a Case ReportMaced J Med Sci 2013 6(1):74-8. [Google Scholar]
[11]. Allison K, Clarridge JE 3rd, Long-term respiratory tract infection with canine-associated pasteurella dagmatis and neisseria canis in a patient with chronic bronchiectasisJ Clin Microbiol 2005 43(8):4272-74. [Google Scholar]
[12]. Akahane T, Nagata M, Matsumoto T, Murayama N, Isaka A, Kameda T, A case of wound dual infection with pasteurella dagmatis and pasteurella canis resulting from a dog bite- limitations of vitek-2 system in exact identification of pasteurella speciesEur J Med Res 2011 16:531-36. [Google Scholar]
[13]. Sorbello AF, O’Donnell J, Kaiser-Smith J, Infective endocarditis due to Pasteurella dagmatis: case report and reviewClin Infect Dis 1994 18:336-38. [Google Scholar]
[14]. Fajfar-Whetstone CJT, Coleman L, Biggs DR, Fox BC, Pasteurella multocida septicemia and subsequent Pasteurella dagmatis septicemia in a diabetic patientJ Clin Microbiol 1995 33:202-04. [Google Scholar]
[15]. Albert TJ, Stevens DLK, The first case of Pasteurella canis bacteremia: a cirrhotic patient with an open leg woundInfection 2010 38:483-85. [Google Scholar]