Cytotoxic radiotherapy, chemotherapy or both are becoming increasingly effective for treating malignancies but are associated with long and short term side effects. The disruption in the function and integrity of the oral cavity is among the clinically important side effects which is acute in nature and may manifest as severe ulceration (mucositis) and fungal infection of the mouth (oral candidiasis, thrush). These disease and treatment induced complications may lead to pain or oral discomfort, poor nutrition, delays in cancer treatment, increased hospital stays and costs and life threatening infection such as septicaemia in some cases [1].
Oral mucositis has now emerged as a major complication of cancer related therapy due to the advent of newer treatment modalities addressing other commonly occurring complications namely nausea, vomiting and neutropenia and thus has become a prominent cause of treatment delay [2,3].
Chlorhexidine gluconate is one of the most commonly used mouthwash solutions identified in studies and has been used in prevention and amelioration of signs and symptoms of both chemotherapy and radiotherapy induced mucositis. However, research does not support much of its use in treatment of mucositis in terms of potential side effects due to the amount of stinging and dehydration it can cause which might lead to microbial colonization over the affected mucosa and further complicate the patients’ condition [2,4]. Also, previously performed study indicates that alcohol containing mouth rinses cause more patient pain than those without alcohol [5]. Thus, it is essential to find a suitable alternative which is more efficacious with minimal side effects.
Phytochemicals have greatly attracted the attention of researchers in the field of medicine. Curcumin, an extract of turmeric is progressively being studied for its varied therapeutic properties such as antioxidant, analgesic, anti-inflammatory, antitumoral, antimicrobial, antiseptic, chemosensitizing and radiosensitizing properties [6–8].
In this back drop, the present study was conducted to evaluate the efficacy and safety of topical curcumin in reducing the severity of signs and symptoms of radio-chemotherapy induced oral mucositis in cancer patients.
Materials and Methods
The study was conducted from August 2014 to October 2014 at Regional Oncology Hospital, Mysuru. Following ethical approval from the Institutional Ethical Committee, 20 cancer patients undergoing radio-chemotherapy and suffering from oral mucositis were selected and randomly divided into the Study and Control groups (10 patients in each group).
Inclusion Criteria
Adult patients diagnosed with Oral Mucositis following radio-chemotherapy for cancer and those willing to be a part of the study and provide a signed informed consent.
Exclusion Criteria
Patients who were terminally ill, using any prophylactic or therapeutic mouthwashes or those unable to comply with curcumin mouth wash as judged by the patient himself or investigator.
Patients were briefed about the study parameters and signed informed consent was obtained. They were randomly assigned into two groups, namely the Study and Control groups consisting of ten patients each.
The study group was given freshly prepared curcumin mouthrinse 0.004%, scientifically prepared under the expertise of Department of Pharmaceutics, College of Pharmacy, to be used in 1:5 dilution for 1 minutes, three times daily for twenty days. The control group was given commercially available chlorhexidine mouthwash 0.2% to be used for 1 minute, in 1:1 dilution, thrice daily for twenty days.
Baseline pain score was recorded using a 10-point scale, the Numerical Rating Scale (NRS), wherein 0=no pain and 10=worst possible pain [9].
The atrophic and erosive changes were quantified based on severity and the number of oral mucosal sites involved. Erythema and ulceration were recorded using Oral Mucositis Assessment Scale (OMAS) [10].
An intensity score for erythema ranging from 0 to 2 was used where,
Grade 0 = normal,
Grade 1= not severe,
Grade 2 = severe
The score for ulcerations was based on area of ulceration ranging from 0 to 3 [10]:
Grade 0= normal,
Grade 1 = Less than 1 cm2,
Grade 2 = between 1-3 cm2,
Grade 3 = ≥ 3 cm2.
The scores for erythema and ulceration were obtained by summing the respective scores for these 16 sites and the total score for clinical signs was obtained by summing the erythema and ulceration scores.
WHO Mucositis scale was used for assessment of oral mucositis in terms of degree of severity of mucositis affecting oral intake of food. The score ranges from 0 to 4 which includes [2,11,12].
Grade 0: No changes.
Grade 1: Soreness / (+) erythema.
Grade 2: Erythema (++), Ulcer, can eat food. (Erythema with ulcers less than 1 cm)
Grade 3: Ulcer (+++), (erythema with ulcers more than 1cms) require liquid food.
Grade 4: Ulcer with haemorrhage and necrosis, alimentation not possible.
The patients were followed up every 10 days. Clinical photographs were taken and the NRS, OMAS & WHO scores were recorded at each follow up visit. A single calibrated examiner assessed the grades. Calibration was done with intent to reduce intraexaminer variability.
Statistical Analysis
The data was entered into SPSS software for windows (version 16.0) and results were obtained. The null hypothesis for the current study was that there would not be any differences between the study and control groups in the onset and severity of oral mucositis. At baseline, all the parameters were tested for randomisation between two groups using independent samples – t-test and all the obtained t-values revealed non-significant differences between study and control groups for NRS, E, U and WHO scores. Later, repeated measures ANOVA were applied to verify the differential changes between study and control groups from baseline to second follow-up.
Results
A total number of 20 patients were included who fulfilled the eligibility criteria. The study group consisted of 10 patients-5 males (50%) and 5 females (50%) [Table/Fig-1]. The control group consisted of 10 patients–6 males (60%) and four females (40%) [Table/Fig-1]. The treatment plan for malignancy consisted of a radiation dosage of 65-70 Gy in 33-35 divided fractions over a period of 6-7 weeks and chemotherapy using injection cisplatin, carboplatin or taxol weekly for a period of 5 weeks.
Shows the demographic data of the study and control groups.
| Patient characteristics | Study group Curcumin mouth rinse n = 10 | Control group Chlorhexidine mouthwash n = 10 |
|---|
| Age | 39 – 70 years (mean 60) | 40 – 71 years (mean 59) |
| GenderMalesFemales | 5 (50%)5 (50%) | 6 (60%)4 (40%) |
| Site of cancerOral cavityPharyngealLaryngeal | 730 | 442 |
| Total radiation dose | 2100 cGy in 15 divided fractions over a period of 3 weeks | 2100 cGy in 15 divided fractions over a period of 3 weeks |
| Total number of chemotherapy cycles received | 2-5 cycles(1 cycle/ week) | 2-5 cycles(1 cycle/ week) |
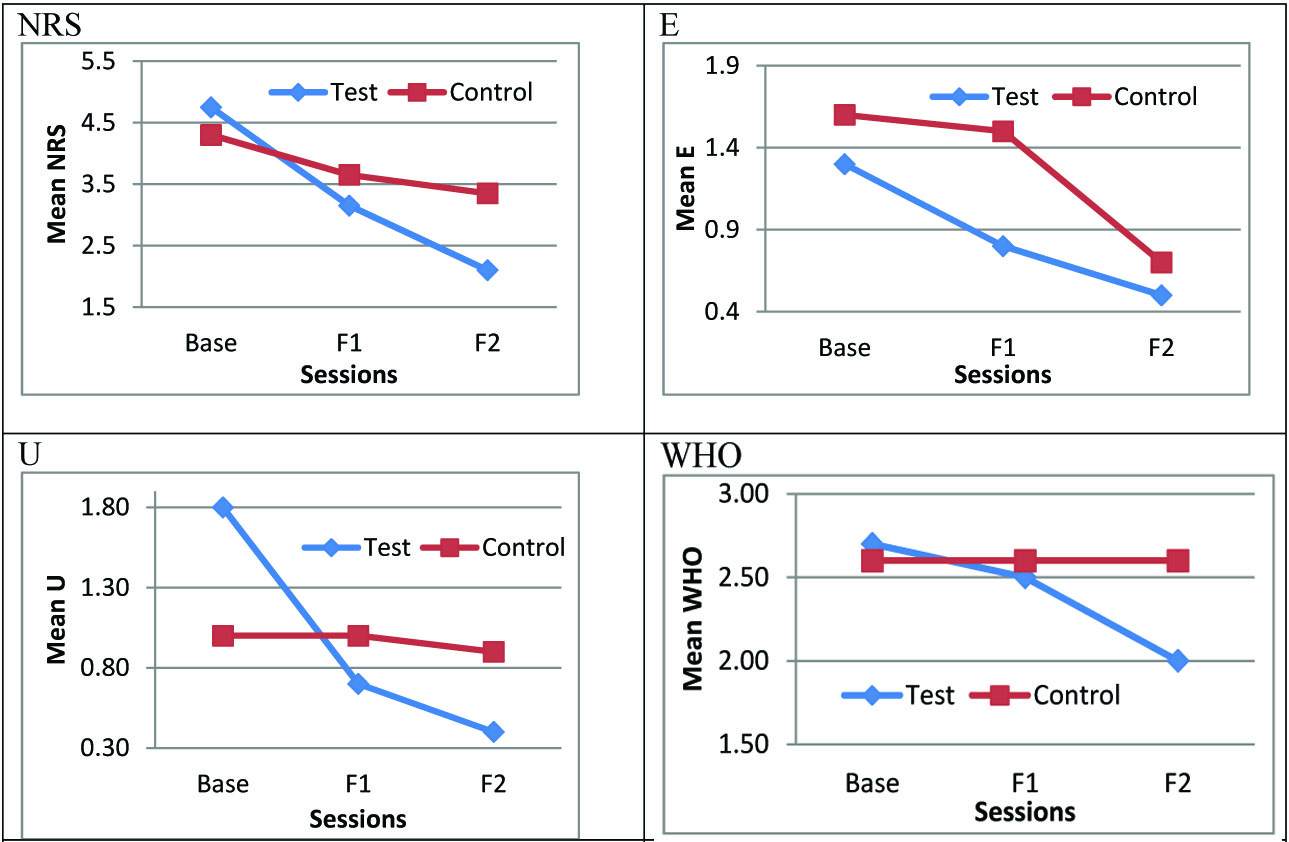
On comparison between the baseline and 2nd follow up scores of the study and control groups, NRS (p<0.001), E (p= 0.050), U (p<0.001) and WHO (p= 0.003) were found to be statistically significant. [Table/Fig-2,3] show the comparison of parameters between the two groups.
Mean baseline, first and second follow up values for various parameters of test and control groups and results of Repeated measure ANOVA
| Variable | Group | Base Line | First follow up | Second follow up | Change | Test statistics |
|---|
| | Mean | ±S.D | Mean | ±S.D | Mean | ±S.D | | |
| NRS | Test | 4.75 | 2.51 | 3.15 | 2.40 | 2.10 | 2.17 | 2.65 | F=11.53;p<0.001 |
| Control | 4.30 | 2.30 | 3.65 | 2.22 | 3.35 | 2.25 | 0.95 |
| Total | 4.53 | 2.35 | 3.40 | 2.27 | 2.73 | 2.24 | 1.80 |
| E | Test | 1.30 | 0.48 | 0.80 | 0.63 | 0.50 | 0.53 | .50 | F=3.26;p=0.050 |
| Control | 1.60 | 0.52 | 1.50 | 0.53 | 0.70 | 0.48 | .70 |
| Total | 1.45 | 0.51 | 1.15 | 0.67 | 0.60 | 0.50 | .60 |
| U | Test | 1.80 | 0.79 | 0.70 | 0.95 | 0.40 | 0.52 | .40 | F=13.78;p<0.001 |
| Control | 1.00 | 0.94 | 1.00 | 0.94 | 0.90 | 0.74 | .90 |
| Total | 1.40 | 0.94 | 0.85 | 0.93 | 0.65 | 0.67 | .65 |
| WHO | Test | 2.70 | 0.48 | 2.50 | 0.97 | 2.00 | 0.94 | 2.00 | F=6.882;p=0.003 |
| Control | 2.60 | 0.52 | 2.60 | 0.52 | 2.60 | 0.52 | 2.60 |
| Total | 2.65 | 0.49 | 2.55 | 0.76 | 2.30 | 0.80 | 2.30 |
Mean baseline, first and second follow up values for various parameters of test and control groups.
NRS – Numerical Rating Scale, E – Erythema, U – Ulceration, F1- 1st follow-up, F2 – 2nd follow-up
Discussion
Oral mucositis varies greatly in severity ranging from mild erythema, which produces burning mucosal discomfort, to large areas of deep, coalescing ulcers that require high doses of opioids for effective intervention. The oral mucosa has an environment rich in microbes such as bacteria, fungi, and viruses. The presence of disruptions provides an important portal of entry for these infectious organisms within the mucosal lining, especially in cancer patients suffering from neutropenia. The importance of mucositis as a risk factor for bacteremia and sepsis is also well established. These factors lead to break from radio-chemotherapy and under treatment resulting in suboptimal cancer therapy [13].
For patients receiving fractionated radiation or chemo radiation for cancers of the head and neck, adverse mucosal changes become apparent at cumulative radiation doses as low as 10 Gy. In almost all cases, ulceration is seen by 30 Gy (the end of the third week of treatment) [13].
A similar observation was noted in our study as well where the signs and symptoms of oral mucositis became clinically apparent in the 3rd week of radiotherapy, i.e. a cumulative dose of 21Gy in 15 divided fractions over a period of three weeks [Table/Fig-1].
Chemotherapy-induced mucositis typically begins 4 to 5 days following infusion and peaks about 5 days later [13]. In our patients, oral mucositis was usually noted in the 2nd week of chemotherapy and the severity of which increased as the number of cycles increased. This change was however, noted more in the control group than in the study group as shown in [Table/Fig-2&3].
The historic paradigm explaining the mechanism of oral mucositis was based on the assumption that cytotoxic treatments intended to kill rapidly dividing cancer cells would also kill rapidly dividing normal cells, such as those found in renewing epithelium. In the case of gastrointestinal mucosa like that in the mouth, therapy induced nonspecific DNA damage to the normal basal cells would cause clonogenic cell death, leading to an imbalance in the equilibrium of epithelial loss and replenishment. When sufficient atrophic damage occurred to thin the epithelium to the point of breaking, ulceration would occur. These ulcers were then unable to heal until the treatment was reduced or stopped. Bacterial colonization and secondary infection of the ulcers were viewed as contributing to their development, duration and healing. Consequently, attempts were made to cure mucositis by approaching the condition as having an infectious aetiology, but these approaches were of little or no benefit.
Chlorhexidine gluconate is a widely used drug in dentistry, which forms a protective barrier over the damaged mucosa consisting of a whitish membrane that results from the coagulation of serum and salivary proteins, thus reducing the severity of oral ulcerations [14]. In view of this property, various studies were performed by several investigators to assess the efficacy of chlorhexidine in management of oral mucositis. In the studies performed by Ferretti et al., and Spijkervet et al., little or no reduction of mucositis was observed in patients receiving high-dose head and neck radiotherapy when chlorhexidine was used as a mouthrinse [15,16]. Foote et al., in their study, showed slightly more amount of stomatitis and adverse effects in patients using chlorhexidine [17], thus ruling out the possibility that chlorhexidine can lower the average daily mucositis score. Epstein et al., observed that there was little effect on lactobacillus count following use of chlorhexidine mouthwash in patients receiving cancer radiotherapy [5,18].
Our study also showed results similar to previously quoted studies wherein, statistically significant difference was noted between the study and control groups in terms of NRS (p< 0.001), E (p = 0.05), U (p< 0.001) and WHO (p= 0.003) scores [Table/Fig-2,3].
The NCCN (National Comprehensive Cancer Network) Task Force on Prevention and Management of Mucositis in Cancer Care reviewed additional published data integrated with expert opinion to produce a comprehensive approach to the management of mucositis and recommended that alcohol containing mouthwashes should be avoided as they cause stinging and dehydration which might lead to microbial colonization over the affected mucosa thus, complicating the patients’ condition further [2].
Recent hypotheses however suggest that infection is neither causative of nor central to the development of oral mucositis [13]. New evidence reveals a more complicated picture of the sequence of events leading to oral mucositis which prompted the investigators to search and provide novel concepts of treatment.
Sonis has proposed a model to characterize the major steps in its development and resolution [19]. In the initiation phase, reactive oxygen species (ROS) generated by exposure to chemotherapy or radiation therapy result in DNA strand breaks and damage to cells, tissues, and blood vessels, which ultimately cause apoptosis. Transcription factors like nuclear factor kappa β (NF-κ β) are activated which leads to signalling and amplification through gene upregulation. Several pathways leading to the damage of epithelial cells and fibroblasts are triggered by cytokines like interleukin (IL)-1β and IL-6. The activity of NF-κ β is amplified by proinflammatory cytokines such as tumor necrosis factor alpha (TNF-α) which in turn promotes inflammation, pain and functional impairment. Colonization of oral bacteria is favoured in the ulceration phase due to penetration of epithelium into the submucosa. All these stages of pathogenesis of mucositis occurs in a continuous manner, with no demarcation between these stages. These stages are triggered in each cycle of chemo-radiotherapy, which leads to a cascade of dynamic interactions repeatedly between treatment cycles occurring at different sites of oral mucosa [2,20,21] .
Aggarwal et al., reported that curcumin has the potential to inhibit NF-κB. The expression of several genes that are regulated by NF-κB has also shown to be suppressed by curcumin [21]. These include cell surface adhesion molecules, chemokines, TNF, MMP9, COX2 & NOS. Since these genes are critical regulators of inflammation, the suppression of expression of these genes explains the anti-inflammatory effects of curcumin [22].
Land et al., performed a study on Role of curcumin and the inhibition of NF-κB in the onset of methotrexate-induced mucosal barrier injury in intestinal mucosa of rats and concluded that inhibition of NF-κB does not increase intestinal side effects of the anticancer treatment, suggesting a safe use of curcumin and caffeic acid phenethyl ester (CAPE) in combination with anticancer treatment [22].
Elad et al., performed a pilot study on 7 paediatric patients receiving chemotherapy to evaluate the efficacy of curcumin oil in controlling the signs and symptoms of oral mucositis and concluded that curcumin mouthwash is well tolerated and efficacious [23]. A similar observation was noted in our study which showed significant improvement in NRS, erythema and ulceration scores. This also explains the significant amount of improvement in the WHO scores as well in the study group.
The therapeutic properties of curcumin in terms of inhibition of growth of various bacteria, fungi and parasites were reviewed by Nagpal et al., [7] and they concluded that curcumin was effective against such microbes. It was observed that following one week application of curcumin, the lesions of guinea pigs infected with dermatophyte and fungi showed improvement.
In the present study, 4 patients (40%) out of 10 in the control group developed oral candida infection as a further complication of oral mucositis. No, such adverse event was noted in the study group thus, providing further evidence in support of the antifungal effect of curcumin.
Limitation of The Study
Considering the small sample size of the study due to time constraints, it is suggested that further studies be performed with a larger sample size to establish the role of topical curcumin in management of radio chemotherapy induced oral mucositis.
Conclusion
Statistically significant difference was found between the Study and Control groups in morbidity associated with Radio-chemotherapy induced OM patients. With the increasing incidence of cancer cases, radio-chemotherapy is inevitable in the treatment of cancer patients, thereby leading to increased morbidity due to associated side effects such as oral mucositis. Thus it is imperative to find a suitable medication that may help improve the quality of life.
Due to the complex multi-factorial patient and treatment factors related to oral mucositis, there is much disparity in assessment and management of oral mucositis and few studies have focussed on oral mucositis as a specific outcome. There is not much evidence in support of the bewildering number of treatment options available presently for the management of oral mucositis. On this note, the current study was performed to evaluate the efficacy of the much studied curcumin in management of innumerable diseases and to exploit its therapeutic potential in the management of radio-chemotherapy induced oral mucositis. Thus, this study concludes that, curcumin mouthwash is well tolerated and effective in controlling the signs and symptoms of Radio-chemotherapy induced oral mucositis.