Ethionamide: Unusual Cause of Hypothyroidism
Ajay Raj Mallela1, Rohini Koya2, Shivashankara Kaniyoor Nagari3, Aswini Kumar Mohapatra4
1 Junior Resident, Department of Internal Medicine, Kasturba Medical College, Manipal, India.
2 Junior Resident, Department of Internal Medicine, Kasturba Medical College, Manipal, India.
3 Professor, Department of Internal Medicine, Kasturba Medical College, Manipal, India.
4 Professor, Department of Pulmonary Medicine, Kasturba Medical College, Manipal, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Ajay Raj Mallela, Department of Internal Medicine, Kasturba Medical College, Manipal, India.
E-mail: ajayprakashdr@gmail.com
Multidrug-Resistant tuberculosis (MDR TB) is major health hazard to the people living in developing countries. As incidence rate of MDR TB has gone up, its therapy has become crucial. MDR TB therapy is known to cause multiple adverse effects however the data related to them is minimal. Hypothyroidism is one of the important adverse effects which usually manifests with vague symptoms and is frequently missed. We present a case of 24-year-old woman who was diagnosed to have MDR TB and started on ethionamide based regimen for same. After 6 months of therapy the patient had clinical symptoms suggestive of hypothyroidism, laboratory investigations confirmed it. As ethionamide is an integral component of MDR TB therapy it was continued and thyroxine replacement therapy was given with which she improved. Hypothyroidism completely resolved after 2 months of stoppage of MDR TB therapy suggesting the reversible aetiology of ethionamide.
Multidrug-resistant tuberculosis, Thyroid stimulating hormone
Case Report
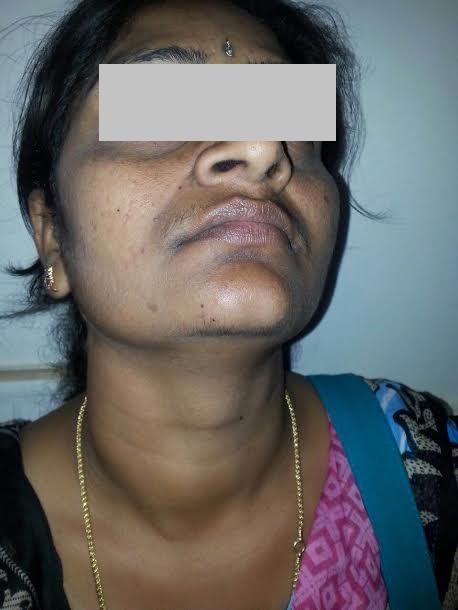
A 23-year-old woman presented to Kasturba Hospital (Department of Medicine) with chief complaints of cough with expectoration. She was diagnosed to have pulmonary tuberculosis 7 years back at local hospital in 2006, was treated as Category 1 with directly observed short course therapy (DOTS) for 6 months. Patient had relapse 3 years later (3 samples showed sputum positivity for acid fast bacilli) and was treated as Category 2 with DOTS in 2009 at Kasturba Hospital for 8 months and was declared cured. Two samples showed sputum positivity for acid fast bacilli diagnosed as Mycobacterium Tuberculosis). Sputum culture (BACTEC MGIT 960) showed growth of Mycobacterium tuberculosis. Drug susceptibility testing (BACTEC MGIT 960) panel included following drugs (Streptomycin, isoniazid, rifampicin, ethionamide and pyrazinamide) showed tubercular bacilli resistant to isoniazid and rifampicin, so diagnosed as MDR tuberculosis. As per WHO guidelines, patient was kept on MDR TB therapy (pyrazinamide, kanamycin, cycloserine, ethionamide, levofloxacin). Intensive phase was planned for 8 months which included kanamycin, pyrazinamide, ethionamide, levofloxacin, para-aminosalicylic acid (PAS). PAS was stopped immediately after starting therapy as patient had an adverse skin reaction to the drug. Cycloserine was added as second bacteriostatic drug in place of PAS. After 6 months of therapy she presented to the hospital with complaints of tiredness, fatigue, menorrhagia, constipation and swelling on anterior aspect on neck for a duration of 2 months which was gradually increasing in size. Examination showed dry coarse skin, puffiness of face, butterfly shaped swelling on the anterior aspect of the neck suggestive of thyromegaly [Table/Fig-1]. Respiratory system examination showed bilateral infrascapular crackles and bronchial breathing in right suprascapular and infraclavicular areas. Laboratory reports including complete blood picture, renal function and liver function tests were within normal limits. Her serum TSH level prior to MDR TB therapy was 1.080 μ IU/ml (0.3-4.5 μ IU/ml) 6 months after therapy TSH was 60.780 μ IU/ml (0.3-4.5 μ IU/ml), T4 was 2.97 μg/dl (5.56-12.2 μg/dl), T3 was 1.18ng/ml (0.8-2.0ng/ml), free T3 was 2.6pg/ml (2.5-3.9pg/ml), free T4 was 0.5ng/ml (0.61-1.12ng/ml). Patient was started with low dose thyroxine therapy 25 μg once daily dosing. Patient had significant improvement with low dose thyroxine therapy. After 1 month of follow up TSH was 26.06 μ IU/ml (0.3-4.5 μ IU/ml). Dose was increased to 50mcg, at 3 months TSH was 8.1μIU/ml (0.3-4.5 μ IU/ml). TSH levels during follow up at 6 months became normal. Ethionamide was planned for duration of 20 months and thyroxine supplementation was planned to give till the end of MDR TB therapy.
A 23-year-old female showing butterfly shaped swelling on the anterior aspect of the neck suggestive of thyromegaly
Discussion
MDR TB has become a global epidemic affecting the population of low socioeconomic status. Drug therapy for MDR TB is of prime importance as WHO highlighted increase in incidence of MDR TB in different regions all over the world [1]. MDR TB is defined as bacillary resistance to isoniazid and rifampicin. Ethionamide induced hypothyroidism was reported first in early 1960s, since then it was reported rarely in literature [2,3]. MDR TB therapy should include at least four second-line anti tuberculosis along with pyrazinamide during the intensive phase of therapy. The drug regimen should include pyrazinamide, a parenteral agent, a fluoroquinolone, ethionamide and cycloserine or PAS if cycloserine is has a contraindication for its use. The total duration of therapy is for 20 months with intensive phase for 8 months which is recommended in many patients [4]. MDR TB is treated with 2nd line agents that includes ethionamide, most likely agent which caused hypothyroidism in the present patient. The treatment regimen suggested by RNTCP for MDRTB cases in India appears good in the context of high cure (66%) and low death (8%) [5]. Ethionamide is not considered as 1st line therapy because of its significant gastrointestinal side effects. Ethionamide is thought to prevent organification of iodine in thyroid hormone synthesis as it is structurally related to other thionamides (propylthiouracil and methimazole) [2]. Other MDR TB therapy drug that can cause hypothyroidism is Para-amino salicylic acid (PAS). As the present patient had early adverse reaction to PAS (Naranjo’s adverse drug reaction probability scale score was more than 9) [6] it was stopped. Satti H et al., reported that when 186 patients who were taking MDR TB therapy were screened for hypothyroidism, 69% had documented evidence of hypothyroidism (cut of TSH >10 mIU/l) with median time to hypothyroidism was 65 days [7]. It is clearly evident and important to monitor TSH in patients on MDR TB therapy from above study [7]. Symptoms based testing of TSH will result in under diagnosis of hypothyroidism in patients on MDR therapy [7].
WHO guidelines recommend screening of TSH 6-9 months after starting therapy and Indian National Guidelines advice TSH screening only when patients are symptomatic [8,9]. It is advisable to screen all the patients within 2-3 months after starting MDR TB therapy followed by screening at 6 months till proper guidelines for screening of hypothyroidism are established. Regular monitoring of thyroid function is recommended in all patients on MDR TB therapy on long term basis Hypothyroidism may manifest with non specific symptoms so it is easily overlooked attributing symptoms to the therapy [7], which affects the patient’s compliance. Even subclinical or mild hypothyroidism should be treated as it causes depression and adherence to therapy will be affected [10]. Patients who are diagnosed to have ETH/PAS induced hypothyroidism should be treated with thyroxine replacement therapy, either of drugs should not be stopped. Thyroid function usually optimises within 2 months after stopping MDR therapy [11]. In accordance with other case reports and studies we advise TSH monitoring in all patients who are on MDR TB therapy [2,3,12,13]. Tuberculosis is regarded as disease of poverty and in resource limited nations it is difficult to monitor TSH levels as most of people belong to poor socioeconomic status. So, simple, faster, affordable tools to measure TSH are in need. This case is a unique as it is highly essential for all treating clinicians to be aware of similar cases of hypothyroidism which is a easily diagnosed and managed entity.
Conclusion
This case highlights the importance of thyroid stimulating hormone (TSH) testing in patients started on MDR TB therapy. It shows symptoms, signs, investigations and management, which would work as a reminder for clinicians. Hypothyroidism is an easily treatable entity that is under diagnosed in patients who are on ethionamide based MDR TB therapy. Symptom based approach for TSH testing are subjected to under diagnosis of hypothyroidism so TSH screening should be done regularly in patients who are on MDR TB therapy till proper guidelines are established. Thyroxine supplementation should be given for hypothyroidism rather than changing the ethionamide based regimen.
[1]. World Health Organization. Towards universal access to diagnosis and treatment of multidrug-resistant and extensively drug- resistant tuberculosis by 2015: WHO progress report 2011. WHO/HTM/TB/2011.3. Geneva, Switzerland: WHO, 2011 [Google Scholar]
[2]. Drucker D, Eggo MC, Salit I E, Burrow GN, Ethionamide- induced goitrous hypothyroidismAnn Intern Med 1984 100:837-39. [Google Scholar]
[3]. McDonnell ME, Braverman LE, Bernardo J, Hypothyroidism due to ethionamideN Eng J Med 2005 352:2757-59. [Google Scholar]
[4]. Guidelines for the programmatic management of drug-resistant tuberculosis 2011 update [Google Scholar]
[5]. Joseph P, Desai VBR, Mohan NS, Fredrick JS, Ramachandran R, Outcome of standardized treatment for patients with MDR-TB from Tamil Nadu, IndiaIndian J Med Res 2011 133:529-34. [Google Scholar]
[6]. Naranjo CA, A method for estimating the probability of adverse drug reactionsClin Pharmacol Ther 1981 30:239-45. [Google Scholar]
[7]. Satti H, Mafukidze A, Jooste PL, McLaughlin MM, Farmer PE, Seung KJ, High rate of hypothyroidism among patients treated for multidrug-resistant tuberculosis in LesothoInt J Tuberc Lung Dis 2012 16(4):468-72. [Google Scholar]
[8]. World Health Organization (WHO) (2008) Guidelines for the Programmatic management of drug-resistant tuberculosis emergency update 2008 (WHO/HTM/TB/2008.402). Geneva [Google Scholar]
[9]. Curry FJ, National tuberculosis center and california department of public health (2008) drug-resistant tuberculosis: a survival guide for clinicianssecond Edition [Google Scholar]
[10]. Yaqoob A, Subclinical hypothyroidism and its consequencesJournal of Public Health and Biological Sciences 2012 1(2):53-60. [Google Scholar]
[11]. Hallbauer UM, Schaaf HS, Ethionamide induced hypothyroidism in childrenSouth Afr J Epidemiol Infect 2011 26(3):161-63. [Google Scholar]
[12]. Andries A, Isaakidis P, Das M, Khan S, High Rate of Hypothyroidism in Multidrug-Resistant Tuberculosis Patients Co-Infected with HIV in Mumbai, IndiaPLOS Published 2013 8(10):e7813 [Google Scholar]
[13]. Dutta BS, Hassan G, Waseem Q, Saheer S, Ethionamide-induced hypothyroidismInt J tuberc Lung Dis 2012 16(1):141 [Google Scholar]