Atrial Fibrillation Due to Over The Counter Stimulant Drugs in A Young Adult
Braghadheeswar Thyagarajan1, Sayee Sundar Alagusundaramoorthy2, Abhinav Agrawal3
1 Resident Physician, Department of Internal Medicine, Monmouth Medical Center, New Jersey, USA.
2 Resident Physician, Department of Internal Medicine, Monmouth Medical Center, New Jersey, USA.
3 Resident Physician, Department of Internal Medicine, Monmouth Medical Center, New Jersey, USA.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Braghadheeswar Thyagarajan, 400, Sairs Avenue, Apt. 21, Long Branch, New Jersey – 07740, USA. E-mail : bragmd@gmail.com
The usage of over the counter stimulant drugs and energy drinks is increasing on a day to day basis for various purposes including work, sports and leisure among individuals in all age groups. Multiple formulations are available in the market including pills, liquid capsules and drinks in various flavours. Many of them contain excessively high doses of caffeine along with a variety of stimulant compounds that have multiple effects in different parts of the human body. The consumption of such high amounts of caffeine itself has shown to have caused cardiac arrhythmias in healthy individuals and when it is mixed with a number of stimulant compounds can be associated with a number of adverse effects in the human body. However, the awareness of such life threatening complications associated with these energy drinks does not exist among people who consume it on a day to day basis. We report a case of 25-year-old Caucasian male with no significant past medical history for cardiac diseases, no risk factors for atrial fibrillation, non smoker, occasional alcohol drinker who presents with new onset atrial fibrillation with rapid ventricular response due to the consumption of over the counter stimulant energy capsule which had high doses of caffeine.
Caffiene, Cardiac arrythmias, Palpitations, Stimulants
Case Report
A previously healthy 25-year-old Caucasian male with past medical history significant only for nephrolithiasis presented to Monmouth Medical Center with complaints of palpitations for the past one day. The patient has no known cardiac history, no other risk factors for atrial fibrillation, non smoker, no illicit drug use, occasional alcohol drinker. Approximately, two days prior to his presentation the patient started consuming an over the counter pre-workout energy supplement [Table/Fig-1]. He stated that he had started to consume this supplement for better concentration and to increase his stamina for his workout. On the day of admission, the patient had gone out for a fishing trip in the morning after consuming another two capsules of the same pre-workout supplement. He developed palpitations and shortness of breath at the fishing trip and was concerned about his symptoms. He presented himself to the Emergency Department (ED) for further evaluation and treatment.
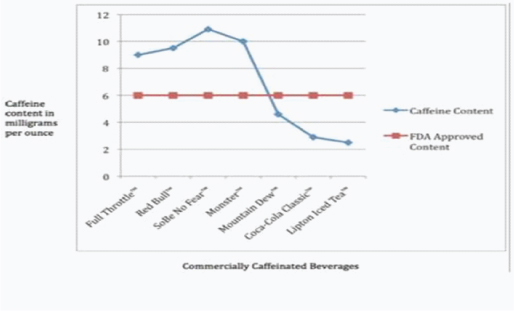
Amount of Caffeine per serving as compared to FDA guidelines [2]
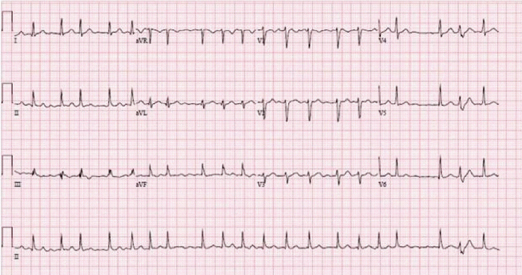
On admission, he denied any further complaints. His vitals were stable except for his rapid heart rate of 140 bpm which was irregularly irregular. He was not in acute distress and his Electrocardiogram showed atrial fibrillation with rapid ventricular response, rate of 123 bpm, normal axis, and no ST-T wave changes [Table/Fig-2]. Physical examination was remarkable for were unremarkable, his thyroid functions were in the normal range, urine and serum toxicology screen were negative for any drugs or illicit substances. Concerning the Electrocardiogram and physical examination findings a diagnosis of new onset atrial fibrillation with rapid ventricular response was made.
Initial EKG at presentation showing atrial fibrillation
The patient was administered intravenous diltiazem boluses in the ED and was started on a diltiazem infusion for rate control of his atrial fibrillation. Heart rate was continuously monitored on telemetry. The patient eventually converted into normal sinus rhythm, 9 hours after initiation of diltiazem infusion. The patient eventually had an echocardiogram which showed LVEF 66%, normal left ventricular size, trace mitral regurgitation and trace tricuspid regurgitation and no other significant valvular pathology. Given the patient’s unremarkable echocardiogram, unremarkable labwork and absent cardiac history along with no other risk factors for atrial fibrillation the cause of his new onset atrial fibrillation was attributed to his consumption of over the counter energy capsules. His CHADS2 VASc score was 0 and hence the patient was eventually discharged home with regular scheduled outpatient follow-up visits.
Discussion
Over the counter stimulants are easily available in the form of pills, energy drink, liquid capsules and energy shots. One of the major constituents of these preparations is caffeine. The amount of caffeine present in these over the counter energy supplements are in excess compared to the standard FDA approved guidelines for quantities’ of caffeine [Table/Fig-1]. These are widely aimed at the teenagers and young adults. Several studies suggest that energy drinks may serve as a gateway to other forms of drug dependence [1].
A number of caffeine formulations are available in the market ranging from regular coffee to energy supplements with concentrated amounts of caffeine, with the content ranging from 50 mg to 505 mg per serving. The estimated average daily adult caffeine consumption is 300 mg. The commonly used energy drinks contain three to four times the caffeine as a typical carbonated beverage and promise to boost performance and enhance metabolism. It is widely believed that the main mechanism of action of caffeine in the central nervous system is the antagonism at the level of Adenosine receptors. Caffeine may also act as a drug of abuse by indirect stimulation of the dopaminergic receptors through removal of the inhibitory effect of adenosine on these receptors. Recent studies have also shown that pre-exposure to caffeine may exert an effect on the classical drugs of abuse [2–4]. Due to this reason caffeine has been considered as an atypical substance of abuse as per DSM 4 TR and DSM 5. Serious toxicities such as seizure and cardiac arrhythmias causing death have been known to be associated with consumption of caffeine at concentrations more than 80 mg/l. The symptoms associated with such complication are usually dizziness, chest pain, shortness of breath, palpitations, syncope and even sudden death [5]. High doses are known to cause cardiac arrhythmias and this risk is increased when the person has underlying structural heart disease [6]. The energy supplement that our patient consumed has around 100 mg of caffeine per pill and the usual dose recommended by the manufacturer is around 2 to 3 pills which adds upto around 200 mg to 300 mg of caffeine.
The various ingredients in our patients’ energy supplement other than caffeine and their studied adverse effects have been shown in [Table/Fig-3]. The ingredients mentioned here are plant extracts with multiple pharmacological effects and clear evidence supporting the use of such products are lacking in the literature. Many of these ingredients have pharmacological properties similar to caffeine and few of them contain caffeine. In addition to the 100mg of caffeine per capsule, if we also consider the added caffeine from these ingredients, the total amount of caffeine per serving (2 to 3 pills) may range from 300 mg to 500 mg of caffeine perserving. If a person consumed this around 2 to 3 times a day the total caffeine consumption in a day may range from 900 mg to1500 mg. The consumption of caffeine in excessive amounts with a mix of such substances with unknown pharmacological effects and unstudied interactions is a major cause of concern. Irrespective of which, consumption of such over the counter stimulant drugs, energy drinks and energy shots containing high amounts of caffeine and other components have shown to have increased risk in cardiovascular complications [6–10].
Common constituents of energy drinks and their pharmacological profile
| Ingredient | Pharmacologic property | Studied effects |
|---|
| Guarana [11] | Similar to caffeine (40mg of caffeine per gram) | Similar to caffeine |
| Synephrine [11] | Long lasting adrenergic effects (sympathomimetic) | Increases systolic pressure with increase in heart rate but no increase in diastolic blood pressure, Unpredictable toxic effects |
| Yohimbine [12] | High affinity to human alpha-2 adrenoceptors, moderate affinity to alpha-1 adrenoceptors, and low affinity to some of the serotonin and dopamine receptors in the central and peripheral nervous systems | Used in erectile dysfunction and causes increased blood pressure, increased heart rate, manic reactions, bronchospasm, palpitations, insomnia, anxiety, irritability, shivering, sweating, nausea, flushing, and headaches |
| Panax [13] | Ginsenosides–affects multiple pathways | Complex effects due to their involvement in multiple pathways– Clear evidence lacking shown to improve psychological functioning Caution advised for use with Warfarin, Oral hypoglycemic agents, Insuline and Phenelzine |
| Evodiamine [14] | Increased 5-HIT promoter activity | Fat burning property with increased heat production Studies limited to animal models, human trials lacking |
| Hoodia Gordonii [15] | Appetite suppressant–Beta Adrenergic receptors | Increased blood pressure and elevated pulserate |
| Octopamine [16] | Moderate affinity to Beta3 adrenergic receptors Poor affinity to Alpha-1, Alpha-2, Beta-1 and Beta-2 receptors | Promote lipolysis and weight loss CNS stimulation |
Treatment of severe caffeine intoxication is generally supportive, providing treatment of the immediate symptoms, but if the patient has very high serum levels of caffeine, then peritoneal dialysis, haemodialysis, or haemofiltration may be required. Management of seizures with benzodiazepenes or barbiturates might also be required. Other managements include replacement of electrolytes and fluids to compensate for fluid and electrolyte losses due to vomiting and prevent renal injury/rhabdomyolysis.
Caffeine in moderate doses is well tolerated and there is therefore no reason to restrict ingestion of caffeine but only when consumed in high doses they become arrythmogenic [17]. Similar to caffeine, other stimulating agents such as recreational drugs, nicotine and alcohol could also lead to potentially dangerous cardiac arrhythmias. It’s common among teenagers to consume drug cocktails in parties such as cocaine, amphetamines, ecstasy which are sympathomimetics and could lead to cardiac arrhythmias [18]. The arrythmogenic effects of alcohol are still unclear. There could be a subclinical heart muscle injury from chronic use causing patchy delays in conduction. Also, the hyperadrenergic state of drinking and withdrawal state, electrolyte abnormalities, impaired vagal heart rate control, repolarization abnormalities with prolonged QT intervals and worsening of myocardial ischemia are the other factors that lead to arrhythmias in heavy alcoholism [19]. Unlike in our patient who is an occasional alcohol drinker, it is clear that his arrhythmia was not secondary to alcohol consumption. While the effect of cigarette smoking on the progression of atherosclerotic diseases is established and well studied, the role of cigarette smoking on cardiac arrhythmia is less clearly defined. The constituents of cigarette smoke such as carbon monoxide and also the oxidative stress caused by them are likely to cause arrhythmias and also, the systemic disease caused due to smoking such as coronary artery disease and also chronic obstructive pulmonary disease, which may lead to cardiac arrhythmias by themselves [20]. Our patient was a non smoker to begin with and hence that could not have been the cause of his arrhythmia.
The follow-up of this patient in 2 weeks after discharge was reassuring without any further complications. It was evident that the patient’s cardiac arrhythmia was triggered due to the consumption of over the counter stimulant energy capsules which had excessive content of caffeine. The patient also refrained himself from the use of over the counter stimulant drugs.
Conclusion
Cardiac arrythmia can be a rare but fatal complication secondary to the consumption of over the counter stimulant drugs containing high amounts of caffeine. Caffeine when present with other ingredients can cause various fatal side effects due to the interactions. Hence, it is advised that manufactuers must clearly state the amount of caffeine per serving in their product, the health risks associated with consumption of caffeine and also mention the side effects due to the additional ingredients.
[1]. Reissig C, Strain E, Griffiths R, Caffeinated energy drinks – a growing problemDrug and Alcohol Dependence 2009 99:1-10. [Google Scholar]
[2]. Garrett B, Griffiths R, The role of dopamine in the behavioral effects of caffeine in animals and humansPharmacology Biochemistry and Behavior 1997 57:533-41. [Google Scholar]
[3]. Nehlig A, Are we dependent upon coffee and caffeine? a review on human and animal dataNeuroscience & Biobehavioral Reviews 1999 23:563-76. [Google Scholar]
[4]. Cauli O, Morelli M, Caffeine and the dopaminergic systemBehavioural Pharmacology 2005 16:63-77. [Google Scholar]
[5]. Banerjee P, Ali Z, Levine B, Fowler D, Fatal caffeine intoxication: a series of eight cases from 1999 to 2009J Forensic Sci 2014 59:865-68. [Google Scholar]
[6]. Ward A, Lipshultz S, Fisher S, Energy drink–induced near-fatal ventricular arrhythmia prevented by an intracardiac defibrillator decades after operative “repair” of tetralogy of fallotThe American Journal of Cardiology 2014 114:1124-25. [Google Scholar]
[7]. Di Rocco J, During A, Morelli P, Heyden M, Biancaniello T, Atrial fibrillation in healthy adolescents after highly caffeinated beverage consumption: two case reportsJ Med Case Rep 2011 5:18 [Google Scholar]
[8]. Klatsky AL, Hasan AS, Armstrong MA, Udaltsova N, Morton C, Coffee, caffeine, and risk of hospitalization for arrhythmiasPerm J 2011 15:19-25. [Google Scholar]
[9]. Sillivent J, Blevins-McNaughton J, Peak K, (2012). Energy Drinks: Ergolytic or Ergogenic?International Journal of Exercise Science 2012 5(3):214-22. [Google Scholar]
[10]. Galemore CA, Sports drinks and energy drinks for children and adolescents–are they appropriate? A summary of the clinical reportNASN Sch Nurse 2011 26(5):320-21.PubMed PMID: 21957570 [Google Scholar]
[11]. Stohs S, Preuss H, Shara M, The safety of citrus aurantium (bitter orange) and its primary protoalkaloidp-synephrinePhytotherapy Research 2011 25:1421-28. [Google Scholar]
[12]. Corazza O, Martinotti G, Santacroce R, Chillemi E, Di Giannantonio M, Schifano F, Sexual enhancement products for sale online: raising awareness of the psychoactive effects of yohimbine, maca, horny goat weed, and ginkgobilobaBio Med Research International 2014 2014:1-13. [Google Scholar]
[13]. Kiefer D, Pantuso T, PanaxginsengAm Fam Physician 2003 68:1539-42. [Google Scholar]
[14]. Hu Y, Ehli E, Hudziak J, Davies G, Berberine and evodiamine influence serotonin transporter (5-HTT) expression viathe5-HTT-linked polymorphicregionThe Pharmacogenomics Journal 2011 12:372-78. [Google Scholar]
[15]. Roza O, Lovász N, Zupkó I, Hohmann J, Csupor D, Sympathomimetic activity of a hoodia. Gordonii product: a possible mechanism of cardiovascular side effectsBioMed Research International 2013 2013:1-6. [Google Scholar]
[16]. Stohs S, Physiological functions and pharmacological and toxicological effects of p-octopamineDrug and Chemical Toxicology 2014 :1-7. [Google Scholar]
[17]. Pelchovitz DJ, Goldberger JJ, Caffeine and cardiac arrhythmias: a review of the evidenceAm J Med 2011 124(4):284-89.doi: 10.1016/j.mjmed.2010.10.017. Review. PubMed PMID: 21435415 [Google Scholar]
[18]. Ghuran A, Nolan J, The cardiac complications of recreational drug useWestern Journal of Medicine 2000 173(6):412-15. [Google Scholar]
[19]. Kupari M, Koskinen P, Alcohol, cardiac arrhythmias and sudden deathNovartis Found Symp 1998 216:68-79.discussion 79-85 [Google Scholar]
[20]. D’Alessandro A, Boeckelmann I, Hammwhöner M, Goette A, Nicotine, cigarette smoking and cardiac arrhythmia: an overviewEur J Prev Cardiol 2012 19(3):297-305.Review [Google Scholar]