Snakebite is a serious problem with an average of 35,000 to 50,000 deaths in India [1]. In a South Indian state like Kerala it is an important medical emergency as well as an occupational hazard. The use of antibiotics is based on either the hospital policies or the primary interventions of the clinicians. The clinician can start an antibiotic based on observation when the diagnosis is unknown, prophylatically to target the organism, empirically when infection is suspected, therapeutically when the organism is suspected and as specific therapy when the organism is known.
There are several conflicting recommendations on the use of antibiotics in snakebite victims [2–6]. While some recommend the routine prophylactic use of antibiotics after snake envenomation [2,3] there are several others who opine that antibiotic usage should be initiated only after clinical evidence of infection such as local tissue necrosis or gangrene [3,4], abscess formation [5], or bullae [6]. Moreover, the sensitivity of the organism and regional antibiotic resistance patterns should guide the selection of antibiotics.
Many types of bacteria both aerobic and anaerobic are known to live in the mouth of healthy snakes and can lead to infections of the bite wound [1,7]. Snakebite wounds should be carefully scrutinized especially in the developing countries because secondary infections may be a complication of some bites, particularly those that develop necrosis [8].
Review of the available evidence suggests that broad spectrum antibiotics like Ciprofloxacin or Amoxicillin+Clavulanate plus Ciprofloxacin or Piperacillin+Tazobactam can be the choices for empirical or definitive treatment, and surgical intervention should be considered for established invasive soft tissue infection [9,10]. The International Classification of Diseases classifies toxic effects of snake venom under T 63.0 [11]. This study was done to identify the pattern of antibiotics used following snake envenomation in a tertiary care hospital of Kerala.
Materials and Methods
Methods
This was a hospital based retrospective descriptive study done from January to August 2011 initiated after getting Institutional Ethics Committee approval. Sampling was census type and all the case sheets filed under T-63.0 of ICD-10 during the study period was manually extracted from the Medical Records Library of the institution and included for data collection. The data was collected in a structured performa. (Appendix 1).
Objectives: To study the pattern of use of antibiotics following snakebite in terms of the type, number, indication, duration, route of administration and dosage level of antibiotics used.
Statistical Analysis
The statistical analysis was done using Statistical Package for Social Sciences-16. Categorical variables like sex, whether patient received antibiotic, the choice of single or combination, the indication, dosage type were summarized as frequencies and percentages and continuous variables like age, duration of antibiotic usage, stay in the hospital, number of antibiotics prescribed, antisnake venom use were expressed as the mean ± standard deviation. The comparison of use of antibiotics with age, sex and type of envenomation was done using chi-square test.
Results
Demography: A total of 313 case records with diagnosis of snake bite were studied of which 58.1% (n=182) were males and 41.9% (n=131) females. Majority of patients fell in the 31-45 years age group as shown in [Table/Fig-1]. The mean age was 37.58± 14.54 years.
Demography of study population
| Age group (in years) | Males (n=182) | Females (n=131) | Total (n=313) |
|---|
| 15-30 | 38 | 69 | 107(32%) |
| 31-45 | 52 | 58 | 110(25.1%) |
| 46-60 | 29 | 39 | 68(21.7%) |
| >60 | 12 | 16 | 28(8.9%) |
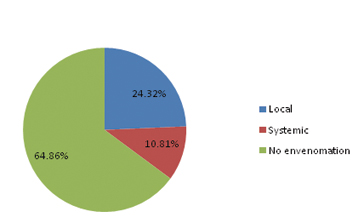
Use of antibiotics: About 94.6% (n=296) received antibiotics, of which 24.32 % had evidence of local and 10.81% had systemic envenomation, while 64.86 % (n=192) were patients with no envenomation as shown in [Table/Fig-2]. There was no age-wise (chi square=3.37, p=0.34) or sex-wise (chi square=0.003, p=0.95) predilection for prescribing antibiotics in patients with snake bite.
Clinical profile of patients who received antibiotics
There were a total of 454 prescriptions of antibiotics with a mean antibiotic usage of 1.46±0.716 per patient. The number of antibiotics prescribed in a patient ranged from 0 to 3 as shown in [Table/Fig-3]. Of the 296 patients who received antibiotics 52.7% (n=156) were prescribed a single antibiotic and the rest 47.3% (n=140) received a combination of 2 or more drugs. The mean duration of antibiotic usage was 3.16±1.446 days. All the patients with local and systemic envenomation and 91.86% of patients with no envenomation received antibiotics.
Number of antibiotics prescribed
| Number of antibiotics | 0(n=17) | 1(n=156) | 2(n=120) | 3(n=20) |
|---|
| Sex | Males(n=182) | 10 | 100 | 66 | 6 |
| Females(n=131) | 7 | 56 | 54 | 14 |
| Age group | 15-30(n=107) | 7 | 59 | 41 | 0 |
| 31-45(n=110) | 3 | 59 | 34 | 14 |
| 46-60(n=68) | 4 | 25 | 33 | 6 |
| >60(n=28) | 3 | 13 | 12 | 0 |
| Envenomation | Local(n=72) | 0 | 32 | 20 | 20 |
| Systemic(n=32) | 0 | 13 | 19 | 0 |
| No(n=209) | 17 | 111 | 81 | 0 |
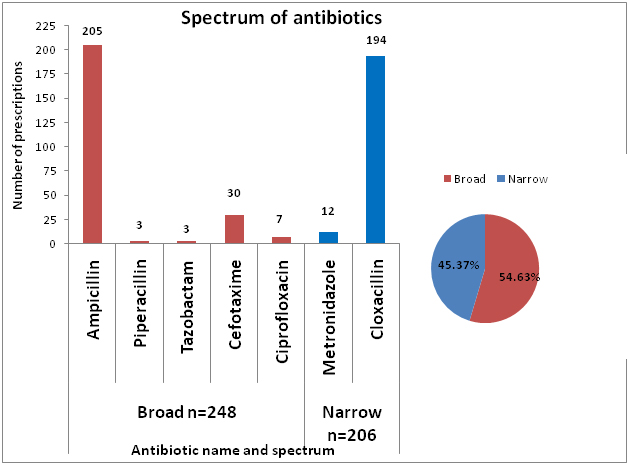
Seven different antibiotics belonging to three different groups Beta lactams, Fluroquinolones and Nitro-imidazoles were prescribed as shown in [Table/Fig-4]. The most widely prescribed antibiotic was Ampicillin closely followed by Cloxacillin. Even though there was no statistically significant (chi-square=5.237, p=0.07) pattern in using single or combination antibiotics with types of envenomation, in patients with no signs of envenomation who received antibiotics, Cloxacillin 63.54% (n=122) was the commonest as shown in [Table/Fig-5]. The most widely prescribed combination was that of Ampicillin with Cloxacillin. The combination of Ampicillin and Cloxacillin was used in doses of 500mg as well as 250 mg each and as shown in [Table/Fig-6] 34 patients who received 250 mg each of Ampicillin and Cloxacillin received suboptimal dose of antibiotics. [Table/Fig-7] shows the spectrum of antibiotics used.
Antibiotics prescribed in snake bite patients
| Classification | Subclass | Antibiotics | Single | Combination | n=454(%) |
|---|
| Beta lactams | Penams | Ampicillin | 74 | 131 | 205(45.15) |
| Cloxacillin | 61 | 133 | 194(42.73) |
| Piperacillin | 0 | 3 | 3(0.66) |
| Tazobactam | 0 | 3 | 3(0.66) |
| Cephams | Cefotaxime | 21 | 9 | 30(6.6) |
| Fluroquinolones | | Ciprofloxacin | 0 | 7 | 7(1.54) |
| Nitroimidazoles | Metronidazole | 0 | 12 | 12(2.64) |
Comparison of use of antibiotics (single and combination) with envenomation
| Use | Antibiotics | Route | Envenomation | Total |
|---|
| Local | Systemic | No |
|---|
| Single (n=156) | Ampicillin | Oral | 20 | 0 | 54 | 74 |
| Cloxacillin | Oral | 12 | 4 | 45 | 61 |
| Cefotaxime | IV* | 0 | 9 | 12 | 21 |
| Combinations (n=140) | Ampicillin + Cloxacillin | Oral | 16 | 19 | 77 | 112 |
| Ampicillin + Cloxacillin + Cefotaxime | Oral+IV | 3 | 0 | 0 | 3 |
| Ampicillin+ Cloxacillin+Metronidazole | Oral | 12 | 0 | 0 | 12 |
| Ampicillin + Ciprofloxacin | Oral | 0 | 0 | 4 | 4 |
| Cloxacillin + Cefotaxime | Oral+IV | 3 | 0 | 0 | 3 |
| Cloxacillin + Cefotaxime + Ciprofloxacin | Oral+IV | 3 | 0 | 0 | 3 |
| Piperacillin + Tazobactam | IV | 3 | 0 | 0 | 3 |
| No(n=17) | No | | 0 | 0 | 17 | 17 |
*IV-intravenous
Antibiotic usage-dose, route and ASV co-administration
| Variables | n=296(%) |
|---|
| Dose | Optimum | 262(88.51) |
| Suboptimum | 34(11.49) |
| Route | Oral | 263(88.85) |
| Parenteral | 24(8.1) |
| Both oral and parenteral | 9(3.04) |
| ASV | Co-administered | 109(36.82) |
| Not co-administered | 187(63.18) |
Spectrum of antibiotics used in patients with snake envenomation
The commonest route of administration was oral (88.85%). Piperacillin+Tazobactam and Cefotaxime were the parenteral antibiotics prescribed. In 36.82% (n=109) patients receiving antibiotics, ASV was co administered; however it was not statistically significant. (Chisquare=0.244, p=0.622)
Summary of clinical profile
[Table/Fig-8] summarizes the clinical profile and management of patients.
Summary of clinical profile
| Variables | 313(%) |
|---|
| First care | Tertiary hospital | 252(80.5) |
| Local hospital | 61(19.5) |
| Site of bite | Lower extremities | 271(86.58) |
| Upper extremities | 39(12.46) |
| Paraspinal | 3(0.95) |
| Type of bite | Venomous | 104(33.22) |
| Non venomous | 209(66.78) |
| Envenomation | Local | 72(23) |
| Systemic-Neurotoxic | 19(6.07) |
| Hemotoxic | 13(4.15) |
| Features -Local Envenomation | Pain and redness | 13 |
| Swelling | 20 |
| Swelling and lymph nodes+ | 11 |
| Cellulitis | 21 |
| Gangrene | 6 |
| Necrotizing fascitis | 1 |
| Features- Systemic Envenomation | Bleeding from site | 7 |
| Facial Puffiness | 2 |
| Hematuria | 4 |
| Delirium and Numbness | 7 |
| Ptosis, Diplopia | 10 |
| Respiratory Paralysis | 2 |
| Concomitant medications & support | Tetanus Toxoid | 247(78.9) |
| ASV | 113(36.1%) |
| Fresh Frozen Plasma, EthamsylateAtropine+Neostigmine, Paracetamol,Diclofenac, Tramadol. Metoclopramide, OndansetronCetrizine, Deriphylline, Ranitidine, Omeprazole,Pantoprazole, Rabeprazole, Glycerine Magnesium sulfate, B complex, Mechanical ventilation | |
| ASV use | Mean usage | 4.65±7.68 vials |
| Mode, Minimum, Maximum usage | 10,2,35 vials |
| Anaphylaxis managed with Pheniramine & Hydrocortisone | 59 |
| Deaths | 5(1.6) |
Discussion
Snake envenomation is a challenging medical emergency and the only available specific antidote is antisnake venom [12]. The use of antibiotics in routine prophylaxis of snake bite victims is controversial yet weighing the risks of secondary infections some researchers have supported the view.
This study done during January to August 2011 included 313 case records with diagnosis of exposure to snake venom. Around 94.6% (n=296) patients received seven different antibiotics supporting the prophylactic use of antibiotics, the choice being made by the treating physicians. This is in concurrence with study done in Zimbabwe by Tagwireyi et al., where 88.5% received prophylactic antibiotics of 10 different types [13]. However, in a prospective study by Blaylock where the protocol was to abstain from antibiotic usage unless evidence of necrosis 84.8% patients did not receive antibiotics [4].
Review of literature shows that cultures of the oral cavities and venoms of different snakes and swabs from necrotic bite sites and abscesses have shown a wide variety of bacteria [1,14–16]. The oral flora of the snakes comprises a wide range of aerobic and anaerobic microorganisms probably because their prey usually defecates while being ingested [16]. The proteolytic properties of snake venom can cause extensive tissue destruction and devitalization, providing an opportunity for the snake’s indigenous flora to infect the bite wound [1].
The bacteria isolated by aerobic culture in a south Indian study included Staphylococcus aureus, Coagulase negative staphylococcus, Enterococcus faecalis, Streptococcus species, Escherichia coli, Klebsiella pneumonia, Proteus species, Morganella morganii, Pseudomonas aeruginosa, Acinetobacter and Enterobacter species but anaerobic culture was not done [1]. Study done elsewhere in the Asian subcontinent suggests that in addition to the aerobic organisms there are also anaerobic organisms like the Bacteroides species and the Clostridium species [8].
Some authors have suggested that before commencing antibiotic therapy culture and sensitivity tests have to be performed [13]. Garg et al., opined that the performance of bacterial culture and antimicrobial sensitivity in each and every patient was a challenge and implementing it was impractical especially in the rural and tribal settings. Hence they suggested that Ciprofloxacin which was frequently prescribed in their study, to which their patients responded well, should be empirically used in patients who develop secondary infection to snakebite in Southeast Asia [1]. In this study, since it was retrospective review the details of culture and sensitivity was not noted down in detail and only seven patients received Ciprofloxacin.
Antibiotics not only prevent dissemination of infection but also accelerate healing [5]. The guidelines for clinical management of snake bite in the South–East Asia region recommends the use of broad spectrum antibiotics like Amoxicillin or a Cephalosporin plus a single dose of Gentamicin plus Metronidazole when there is interference with wound to contain the risk of secondary bacterial infections [17]. Antibiotics like Gentamicin, Tetracycline and Polymyxins should not be ideally used in neurotoxic envenomation as it can accentuate the clinical symptoms owing to its blocking effect on the neuromuscular junction [18]. Review of literature reveals the safe use of gentamicin in a patient with local envenomation who developed cellulitis [19]. The use of tetracycline should be with caution not only in the neurotoxic envenomation but also in the patients with haemotoxic envenomation where it can aggravate acute renal failure [20]. However, this study shows that the antibiotics prescribed in patients with envenomation and no envenomation were Ampicillin, Cloxacillin, Metronidazole, Cefotaxime, Piperacillin+Tazobactam and Ciprofloxacin.
In this study Penicillins were the most frequently prescribed antibiotics, Ampicillin being the commonest. A study from Zimbabwe demonstrated that prophylatically the most frequently used antibiotics were drugs in the Penicillin family [13] Studies from Eastern Ecuador have reported that Ampicillin alone or in combination with another antibiotic was most commonly used for snakebite envenomation [21]. Ampicillin with cloxacillin was the most frequently prescribed combination. The choice of combination is in concurrence with a study by Dhanya et al., however the single most commonly prescribed antibiotic was cloxacillin [12]. Studies done in Hong Kong suggest that most patients receive prophylactic antibiotics following venomous snakebites the commonest regimen being Ampicillin with Cloxacillin [22,23].
The combination was prescribed in a dose of 500 mg each per dose as well as 250 mg each per dose. The use of 250 mg Ampicillin with 250mg Cloxacillin is irrational as it doesn’t provide the required antibiotic concentration in the blood which can tackle the infection in the adults. The next frequently prescribed antibiotic was Cloxacillin which is a narrow spectrum antibiotic. Shek et al., suggests that instead of routine prophylaxis with Ampicillin plus Cloxacillin for established snakebite wound infections Levofloxacin plus Amoxicillin/Clavulanate be used empirically. Levofloxacin plus Metronidazole can be used in patients with Beta lactam allergy as this combination covers most of the aerobic and anaerobic organisms [8]. In this study Ampicillin plus Ciprofloxacin was prescribed in four patients while three patients received Cloxacillin plus Ciprofloxacin along with intravenous Cefotaxime.
A study done in Taiwan suggested that for treatment of minor wounds oral Amoxicillin/Clavulanate plus ciprofloxacin may be considered and for severe wound infections, parenteral Piperacillin/Tazobactam [9]. In this study, three patients with severe local envenomation of which one had necrotizing fasciitis received Piperacillin plus Tazobactam.
Study done by Jorge et al., suggested that Chloramphenicol is a good choice for empirical treatment of soft tissue infections due to snake bite in Brazil, suitable alternatives being Cotrimoxazole or Fluroquinolones along with Clindamycin or Metronidazole [10].
Some authors have raised concern of antibiotic resistance with over use of broad spectrum antibiotics and cited it as the major reason for growing worldwide resistance [13,24]. In this study the use of broad spectrum antibiotics 54.63% where slightly more than the narrow spectrum antibiotics 45.37%. It is notable that majority of persons with no envenomation were prescribed Cloxacillin.
The choice of route of administration of antibiotics was oral alone in 88.5%. Intravenous antibiotics alone or with oral drugs were used only in patients with severe local envenomation. This is in sharp contrast to study done by Tagwireyi et al., where 42.2% of antibiotics administered were parenteral preparations where they attributed the reason for choice as “injections may be viewed as superior treatment than oral medicines in some cultures” [13].
ASV was used in 113 patients and it was co administered in 36.82% (n=109) patients receiving antibiotics however it was not statistically significant. This is in concurrence with study by Chen et al., where the clinical outcome of the patient didn’t vary with antivenom dosage and antibiotic therapy [25]. The 17 patients who did not receive antibiotics would probably have been suspected cases of snake bites with no signs and symptoms of local and systemic envenomation.
Limitations of The Study
Even though chances are rare there might have been misclassification of records or some records might have been missed during manual extraction. Since this is a retrospective analysis data pertaining to culture and sensitivity are missing. The duration of the study is short.
Conclusion
The use of antibiotics in this tertiary care hospital is in concurrence with antibiotic usage elsewhere in different parts of the world. The main pattern of use of antibiotics following snakebite envenomation is Ampicillin alone or in combination with cloxacillin empirically, Cloxacillin prophylatically and Piperacillin with Tazobactam for severe established infections. Since the study setting is in a developing country the prophylactic use of antibiotics following snake bite may be justified weighing the concerns of secondary infections. Prospective studies need to be conducted to correlate the microbiological culture from the wound sites and response to antibiotics as well as cost effectiveness.
*IV-intravenous