Periodontitis is a multi factorial disease of which bacterial plaque is recognized as the main etiologic agent in the initiation and progression of perioodontitis [1]. Removal of bacterial deposit is the fundamental objective of periodontal therapy [2]. The prevalence and severity of periodontal diseases are managed by total reduction of bacterial plaque or at least the pathogenic bacteria.
Conventionally, mechanical subgingival instrumentation namely scaling and root planing has been the main treatment modality in periodontal therapy by which most of the periodontal conditions can be effectively managed. Variation in the efficacy of scaling and root planing to gain access into deep pockets, furcations, root morphological alterations can leave residual plaque deposits in the pocket which can result in the recolonization of the treated areas with pathogenic organisms. This has led to the use of antibacterial agent usually in the form of subgingival irrigants as an adjunct to scaling and root planing.
Sub gingival irrigation has a non-specific action of flushing the pocket contents and thereby it can effectively alter the quality and quantity of unattached subgingival plaque which are associated with chronic perioodontitis [3]. Studies in the past two decades have reported improvements in terms of clinical and microbiological parameters with the adjunct use of subgingival irrigation agents like povidone iodine [4], hydrogen peroxides [5], stannous fluoride [6], boric acid [7], chlorhexidine [8] etc.
Molecular oxygen, hyperbaric oxygen and hydrogen peroxide have been applied as a substitute to conventional irrigation which can hold back the subgingival bacteria within the pocket by modifying the anaerobic subgingival environment [9,10].
Recently, ozone treatment is gaining popularity in dentistry. Ozone (O3) is a gas normally present in the upper atmosphere. The use of ozone has been proposed in dentistry due to its disinfectant, antimicrobial and healing properties. Ozone finds dental application for caries and hypersensitivity treatment, sterilization of cavities and root canals, bleaching, treatment of mucosal lesions, periodontitis, periimplantitis etc.
Periodontal application of ozone is usually done in gaseous, aqueous and oil forms. The use of ozonized water is a safe and simple procedure compared to gaseous ozone. When compared to 2.5% sodium hypochlorite ozonized water have a comparable antimicrobial activity and the fibroblast metabolic activity was high when the cells are treated with ozonized water [11] In most of the situations ozonized water has better biocompatibility than gaseous ozone [12]. Ozonized water subgingival irrigation was used successfully in the treatment of aggressive perioodontitis [13]. In patients undergoing orthodontic treatment, use of ozonized water showed definite improvement in gingival inflammation [14]. The present study is being undertaken to evaluate the use of ozonized water subgingival irrigation as an adjunct to scaling and root planing in patients with chronic generalized periodontitis.
Materials and Methods
A total of thirty (19 males and 11 females) systemically healthy adolescents aged 35-55 years suffering from chronic periodontitis were selected among the patients visiting the Department of Periodontology and Implantology, Mar Baselios Dental College, Kothamangalam. Patients did not receive any surgical and non surgical therapy for last six months and were not in any antibiotic therapy for last six months. The purpose of the study was explained to the patients and written informed consent was obtained and ethical approval was obtained from the institutional ethical committee. For each selected subjects two sites with probing pocket depth ≥6 mm on at least one tooth on contralateral sides of opposite arches were selected.
Proper oral hygiene instruction were given to all patients and in each patient a mandibular site having ≥ 6mm pocket was assigned as the test site and a contra lateral maxillary site with ≥ 6mm pocket was chosen as the control site. After the recording of clinical parameters like Gingival index [15], probing pocket depth and clinical attachment level and microbiologic sampling, all patients underwent full mouth oral prophylaxis using ultra sonic scalers (SATELEC P5 Booster Suprasson, France). SRP was done at the test and control sites using area specific Gracey curettes (Hu xs– Friedy # 1/2, # 11/12, # 13/14) until the root surfaces felt smooth and hard to tactile exploration. After scaling and root planing microbiologic samples were again collected from both test and control sites.
Irrigation Protocol
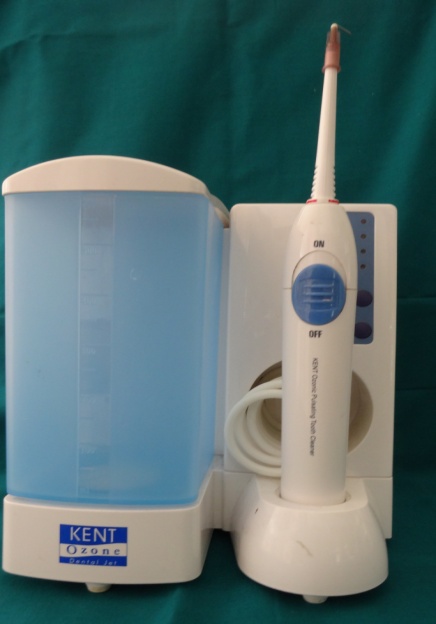
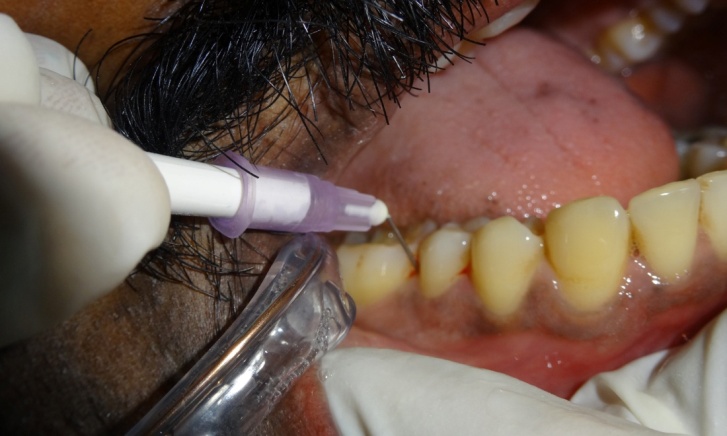
The test sites were subjected to ozonized water subgingival irrigation. The subgingival irrigation device (Kent Ozone Dental Jet TY 820 Kent Ro Systems Ltd, Noida, India) [Table/Fig-1] fitted with a modified subgingival tip was inserted 3mm deep into the pocket [Table/Fig-2]. The pocket was irrigated for one minute. Entire mandibular teeth were irrigated in this manner. The device released a single pulsating stream of ozonized water from the nozzle with a water outflow of ≥150ml/min with ozone output of 0.082 mg/h. Microbiologic sampling were repeated at the test sites. The patients were recalled at the first, second and third weeks. During the recall visits test sites along with the entire mandibular teeth received ozonized water subgingival irrigation in the above mentioned manner and oral hygiene instructions were reinforced. After 4 weeks patients were reviewed and clinical parameters were recorded and microbiologic sampling was done at the test and control sites.
Ozonized Water Irrigation
Collection Of Subgingival Plaque Sample and Microbiological Analysis: Microbiological sampling was done at both test and control sites in the following manner. After removing supra gingival plaque, tooth was isolated with sterile cotton rolls. Three sterile fine endodontic paper points (No. 40) were inserted to the depth of each study periodontal pocket for 30 seconds, and transferred to 10 ml of Thioglycollate media. Microbiologic samples were vortxed in a vortex mixer and subsequently incubated for two hours. The samples were then serially diluted. 100μl of the diluted specimen was spread with a sterile L shaped spreader onto brain heart infusion agar and anerobically cultured using anerobic jar (MERK) with anerobic generator Anacult A (Gas Pack) at 35-37°C for 5 days. All the samples were then inspected for total anerobic colony count, using the digital colony counter.
Statistical Analysis
Statistical analysis was done using inferential statistics such as independent t-test for comparison of changes in clinical and microbiologic parameters between test group (SRP + Ozonized water group) and control group (SRP alone) in the baseline and after 4 weeks. Paired t-test was done to assess the changes within the groups at the baseline and after 4 weeks.
Results
Baseline values showed no statistically significant difference in any of the clinical parameters at the test and control site p-value < 0.01 is considered to be statistically significant. Gingival index, probing pocket depth, clinical attachment level showed statistical significant reduction after 4 weeks in both test and control sites. However, compared to control site, test site showed more significant improvement in Gingival index, probing pocket depth and clinical attachment gain after 4 weeks [Table/Fig-3,4,5,6,7,8 and 9]. The mean anerobic colony count after scaling and rootplaning was 108.23±20.30 in test site and 114±18.95 in control site. After 4 weeks it was reduced to 77.43±13.27 in test site and in control site it was increased to 162.67±15.79. thus there was a statistically significant difference in anerobic colony count between baseline and after 4 weeks in both test and control site, anerobic colony count was reduced by 28.5% in test site and it was increased by 41.8% in control site after 4 weeks [Table/Fig-10,11,12,13 and 14].
Inter site comparison of gingival index
| | Mean | SD± | N | t | p-value |
|---|
| Baselines | Test | 1.88 | 0.33 | 30 | 1.63 | 0.109 |
| Control | 2.00 | 0.21 | 30 |
| 4 weeks | Test | 0.73 | 0.27 | 30 | 5.25** | <0.0001 |
| Control | 1.13 | 0.33 | 30 |
**: - Significant at 0.01 level
Inter sites comparison of probing pocket depth
| | Mean | SD | N | t | p |
|---|
| Baselines | Test | 6.43 | 0.73 | 30 | 1.12 | 0.269 |
| Control | 6.67 | 0.88 | 30 |
| 4 weeks | Test | 3.93 | 1.72 | 30 | 4.7** | <0.0001 |
| Control | 5.67 | 1.06 | 30 |
**: - Significant at 0.01 level
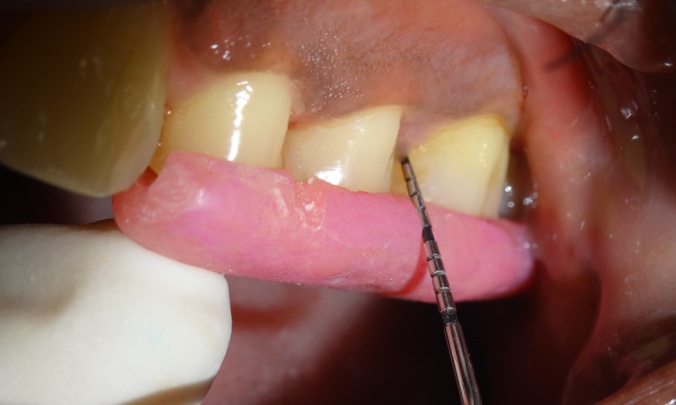
Probing pocket depth at baseline in test site
Probing pocket depth after 4 weeks in test site
Probing pocket depth at baseline in control site
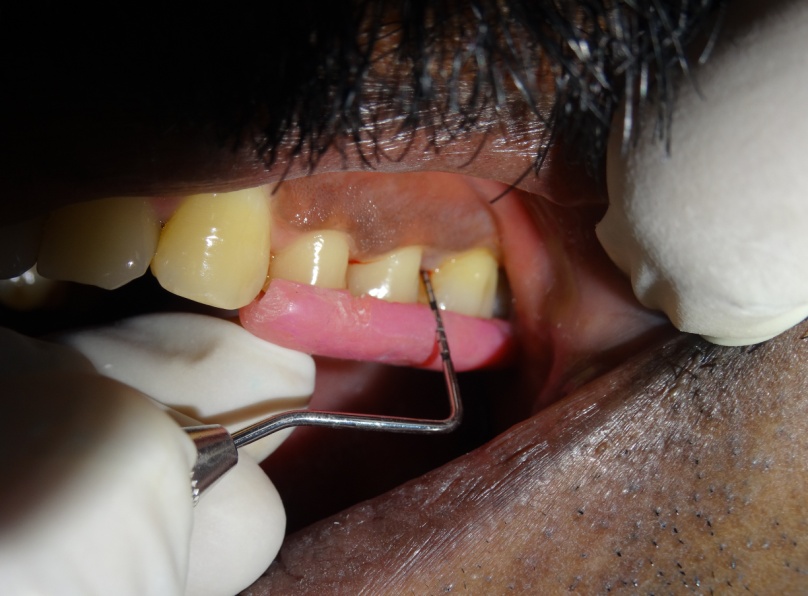
Probing pocket depth after 4 weeks in control site
Inter sites comparison of clinical attachment level (CAL)
| | Mean | SD | N | t | p-value |
|---|
| Baselines | Test | 6.90 | 0.92 | 30 | 0.39 | 0.699 |
| Control | 6.80 | 1.06 | 30 |
| 4 weeks | Test | 4.40 | 1.85 | 30 | 3.38** | 0.001 |
| Control | 5.73 | 1.11 | 30 |
**: - Significant at 0.01 level
Anerobic colony count at baseline in test site
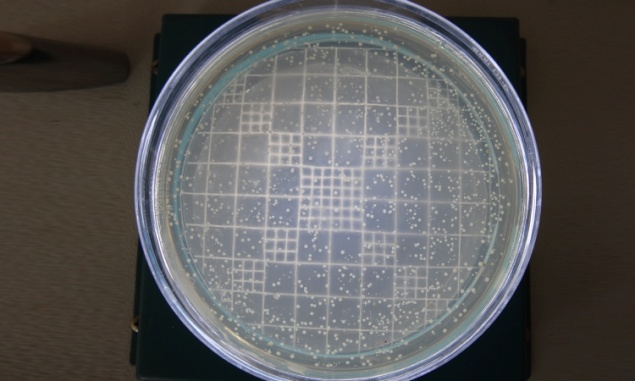
Inter site comparison of anerobic colony count
| | Mean∞ | SD | N | t | p-value |
|---|
| Baselines | Test | 644.10 | 108.63 | 30 | 1.14 | 0.258 |
| Control | 606.20 | 145.63 | 30 |
| After scaling | Test | 108.23 | 20.30 | 30 | 1.28 | 0.205 |
| Control | 114.73 | 18.95 | 30 |
| 4 weeks | Test | 77.43 | 13.27 | 30 | 22.63** | <0.0001 |
| Control | 162.67 | 15.79 | 30 |
**: - Significant at 0.01 level ∞= *103 CFU
Anerobic colony count after 4 weeks in test site
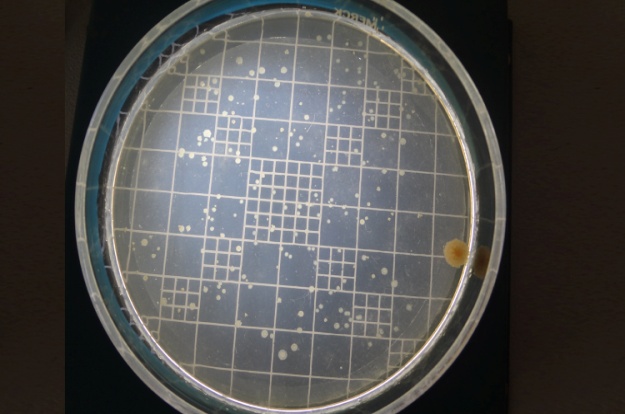
Anerobic colony count at baseline in control site
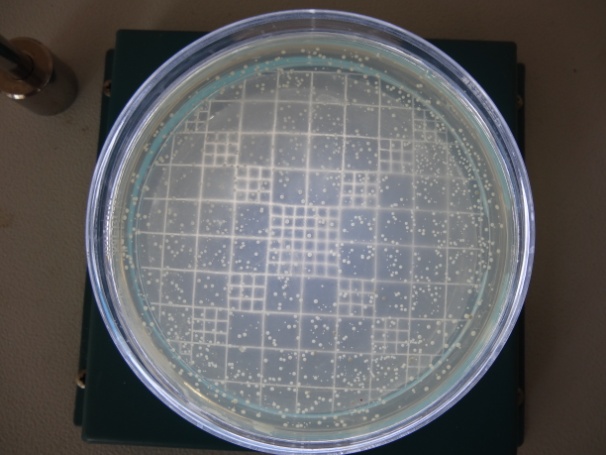
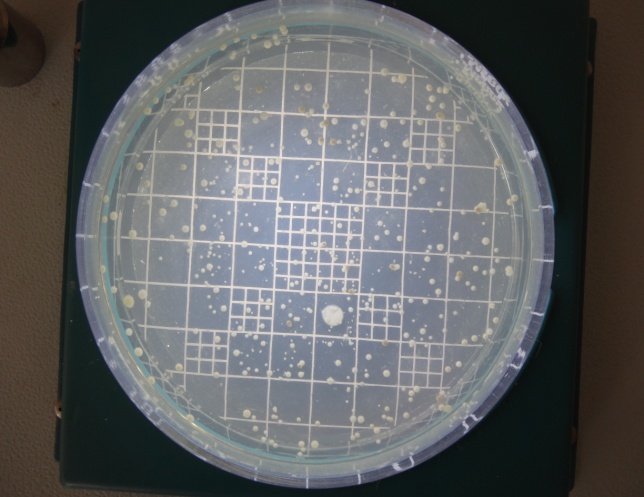
Anerobic colony count after 4 weeks in Control Site
Discussion
The aim of the present study was to compare the clinical and microbiologic benefit of mechanical therapy along with adjunctive use of ozonized water subgingival irrigation in the treatment of chronic periodontitis. Mechanical and chemotherapeutic measures minimize or eliminate bacterial plaque and prevent their recolonization, have been used in periodontal therapy in the past with varying results. Studies by Waerhaug et al., and Rabbani et al., concluded that complete plaque removal by mechanical measures in pocket depth above was 5mm was difficult [16,17], so adjunctive methods that enhance the effect of mechanical measures can be used. Irrigation of periodontal pockets is a commonly employed treatment modality in periodontics which is used as a simple adjunct to mechanical instrumentation. The rationale of irrigation is derived from two basic principles. Mechanical effect, which is the flushing action of the irrigant, was believed to remove the residual biofilm in the pocket and the antimicrobial action of the agent can potentially reduce the bacterial count within the pocket.
Professional subgingival irrigation with antimicrobial agents is generally used as an adjunct to scaling and root planing (SRP). Clinical trials on subgingival irrigation using chlorhexidine, providone iodine, tetracycline, boric acid etc have reported significant improvements in clinical and microbiological parameters of periodontal disease.
Chlorhexidine has disadvantages like propensity to stain teeth and restorations and toxicity for gingival fibroblasts, which can impair periodontal healing and cause mucosal desquamation [18]. Hydrogen peroxide is a strong oxidizer, a compound with an oxygen-oxygen single bond. The use of 3% subgingival irrigation with hydrogen peroxide has failed to demonstrate microbial shifts compared with saline irrigation when used in combination with mechanical debridement [19]. Povidone iodine in 5-10% solutions has an inhibitory effect of the serum and can kill bacteria of experimental biofilm [20] but is not able to do so for all naturally formed biofilm. Therefore, an agent that is biocompatible and has good antiseptic and antimicrobial potential would be useful for periodontal therapy. Ozone has been recently advocated as an irrigating agent predominantly due to its antimicrobial action which results from oxidation of microbial cellular components and altering the sub gingival homeostasis. When ozone dissolves in water Hydroxyl radical is generated and it is highly unstable. The antimicrobial action is by direct reactions of molecular ozone and other a free radical mediated reaction.
Schlangenhauf et al., disclosed the antimicrobial action of ozonized water against the periodontal pathogens like Aggrigatibacter actinomycetemcomitants and Porpyromonas gingivalis [21]. There were results of invitro studies in dental literature reporting the effect of ozonized water against bacteria invading the dentinal tubule [22], E.faecalis [23] and Candida albicans [24]. Previous invitro studies have reported that ozonized water has antibacteraial effect. But in vivo studies supporting the effect of ozonized water were in sufficient hence in the present study along with the clinical parameters microbiological evaluation was also done.
The gingival scores over a period of 4 weeks reduced significantly in test sites compared to the control sites. The improvement observed in Gingival index may be due to reduction in inflammation that may be attributed to antimicrobial property of ozone. The improvement in gingival status was less than that observed by Dodwad et al., [25] (72%), but higher than what was observed by Kshitish et al., [10] (29%). This difference may be attributed to variation in duration of the study.
After 4 weeks of ozonized water irrigation, Hayakumo et al., [26] evaluated a statistically significant mean reduction of 2.24mm and Dodwad et al., [25] evaluated a statistically significant mean reduction 2.5mm (39.68%) in probing pocket depth when compared with the baseline. This result comes in agreement with our study. The present study attained a significant clinical attacment gain of 2.42 mm after 4 weeks which was similar to those achieved in studies by Hayakumo et al., [26] (2.42mm).
Studies have shown that subgingival microbial recolonization occurring over a period of time is a major limitation of mechanical instrumentation leading to the recurrence of disease. In the present study, test sites showed a significant reduction in anerobic colony count immediately after scaling and root planing when compared to the baseline. Test sites also showed a significant reduction in anerobic colony count after 4 weeks when compared with the baseline and immediately after scaling and root planing. The repeated pulsated ozonized water sub gingival irrigation might have interfered with the recolonization of subgingival micro flora. Ramzy et al., and Dodwad et al., [13,25] reported that ozonized water irrigation for a period of 4 weeks showed a significant reduction in microbial flora and Kshitish et al., concluded that periodontal pathogens was reduced in after ozonized water irrigation at baseline and at the 7th day [10].
Control sites showed statistical significant reduction in anerobic colony count immediately after SRP when compared with the baseline. But it showed a statistical significant increase after 4 weeks when compared with scaling and root planing. Since there was no intervention during the 4 weeks interval, there might be recolonization of periodontal pocket. Mosques et al., [27] concluded that after SRP, bacteria recolonize and reach the baseline approximately within 42 days. Greenwell et al., [28] concluded that bacterial colonies recolonized and reached the baseline with in 4-8 weeks. However, when compared with baseline there was significant reduction in bacterial load after 4 weeks. This may be attributed to the improvement in the oral hygiene status of the subjects.
Intergroup comparisons showed that there were no significant differences in the microbial counts of the pocket at baseline between test and control sites. Comparable reduction in the microbial counts were observed immediately following SRP at both test and control sites. Subgingival debridement has been observed to result in a decrease in the total number of micro organisms present in subgingival sites and a shift in the relative proportion of different microbial species within the subgingival plaque biofilm. A decrease in the total bacterial count for sites of 3 mm or greater depth has been observed immediately following subgingival debridement.
Following ozonized water irrigation, the test sites showed a considerable additional reduction in the counts. This was suggestive of the efficacy of irrigation in reducing the microbial load within the pocket over and above SRP. At 4 weeks, there was a mean increase in the microbial counts at both test and control sites; however, at the test sites, it did not reach the immediate post-instrumentation levels. Following treatment, the subgingival habitat may be repopulated by microorganisms which originate mainly from residual subgingival plaque deposits [29].
It can be summarised that the application of ozone at the test sites resulted in the enhancement of the antimicrobial effects of SRP by removing the residual organisms. This greater reduction may be explained on the basis that scaling and root planing can promote the disorganization of the subgingival biofilm thereby reducing the inherent microbial load the irrigant has to act upon (inoculum effect).
Another possible effect of ozone application is the change in the subgingival environment, the relevance of which is highlighted in the ecological plaque hypothesis. Ozone with its potent oxygenating action can provide an aerobic environment within the subgingival domain thereby suppressing the growth of anerobic organisms.
Limitations
There are a few limitations to the current study. Employing placebo irrigation at the control sites would have eliminated the potential effects of the flushing action of the irrigant, thereby elucidating the additional benefits of ozone as a specific agent. Moreover, the more sustained effects of irrigation have been noticed with the concomitant use of home irrigation by the patient and the stability of ozone in water was low and ozone dissipated very quickly at room temperature over 5 minutes [30]. The study did not examine the effects on specific periodontal pathogens that would have been more relevant. Finally, a 4 week analysis may be an insufficient time period to evaluate the long term effects of this modality as a useful adjunct to mechanical instrumentation so further long term studies needed to assess the clinical and microbiological effect of ozonized water.
Conclusion
Within the limitations of this study, we can conclude that ozonized water subgingival irrigation is effective in improving oral hygiene, reducing gingival inflammation, decreasing pocket depth and increasing attachment levels when used as an adjunct to scaling and root planing in patients with chronic periodontitis. Subgingival application of ozonized water along with scaling and root planing significantly reduces the anaerobic bacterial count in periodontal pockets and prevents their recolonization. Further studies utilizing larger sample sizes and longer follow-up periods are recommended for supporting the finding of this study.
**: - Significant at 0.01 level
**: - Significant at 0.01 level
**: - Significant at 0.01 level
**: - Significant at 0.01 level ∞= *103 CFU