Patients attending sexually transmitted infections (STI) clinics are routinely screened for syphilis by employing a battery of serological tests. Venereal disease research laboratory (VDRL) test is a commonly employed screening procedure in this regard. This test measures the non-treponemal or reagin antibodies produced in response to Treponema pallidum.
For any laboratory test, the sequence in which the steps are performed constitutes the “testing cycle” that has been outlined by Lundberg as a series of nine steps: ordering, collection, identification, transportation, preparation, analysis, reporting, interpretation and action [1,2]. An important index that has been defined to quantify the time for this cycle in an objective manner is the “turnaround time” [3]. While the “laboratory turnaround time” has been defined as the time from receipt of the specimen until time of availability of the result, “total turnaround time” is generally calculated from the time the physician requests the test until the time he views the result [4,5]. Timely reporting of laboratory test results is now considered as an important aspect of the clinical laboratory services as are the accuracy and reliability of the test reports generated by them [6]. Turnaround time of a laboratory is indicative of diagnostic responsiveness of the healthcare system of which it is a part. With reference to a laboratory facility undertaking syphilis serology, timeliness of reporting should be of utmost importance since, to limit the spread of syphilis, turnaround time for its diagnosis needs to be significantly reduced. Analysis of turnaround time helps in determining the possible causes of delay, so that an improvement in this time interval can then follow [6].
The present audit was primarily undertaken as an attempt to evaluate the turnaround time of syphilis testing (mainly VDRL test) in the section of serology laboratory and find out the possible reasons for its delay. To the best of our knowledge this is the first such survey of turnaround time of any syphilis serology facility from India. In our attempt to evaluate the VDRL turnaround time, we also tried to understand the flow of samples from the point the clinician ordered the test to the point the report was available to the patients; the time required at each step; and the possible corrective measures that could be adopted. In addition, this study also seeks to describe the patterns of clinical requisitions for syphilis testing in an STI clinic; to assess the frequency of a positive syphilis serology among STI clinic attendees; and to analyse the follow-up rates of VDRL report collection at the STI clinic.
Materials and Methods
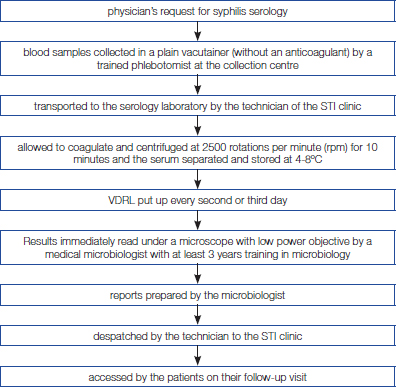
The present analysis is a prospective audit of 200 serum samples received at the serology section of the Department of Microbiology of a tertiary care teaching hospital in New Delhi from the STI clinic of the associated hospital. The serology laboratory of the department receives on an average 15,000 serum samples annually for syphilis serology from both the inpatient and outpatient departments of the linked hospitals. Of these nearly one-tenth of the requests are from STI clinic. The present study was conducted over a two month period during November-December 2013. The VDRL test is generally put up for a batch of more than 150 samples. This requires that the test be put up every second or third day. Any reactive results by the screening assay (VDRL) are further confirmed by treponema pallidum haemagglutination assay (TPHA).
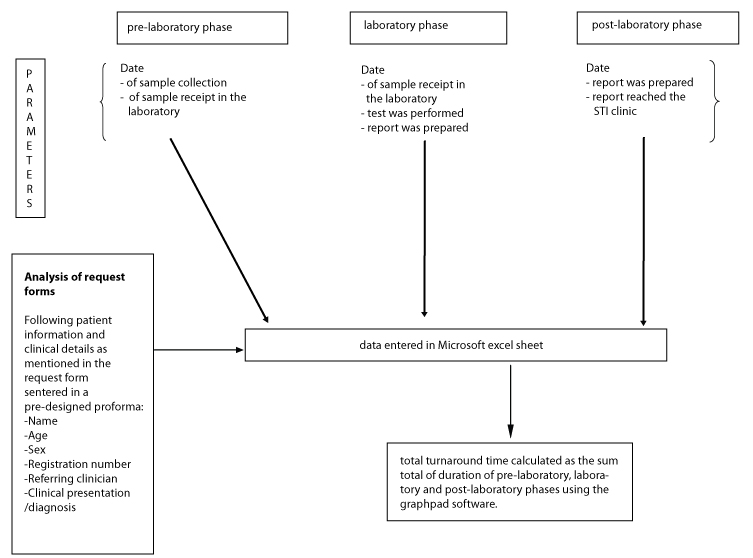
For the purpose of the audit, the time points of workflow were defined as:
pre-laboratory phase: time from clinician request to the point the sample was received in the laboratory; laboratory phase: from the time the sample was received in the laboratory to the time the final report was prepared; and the post-laboratory phase: from the time the report was prepared to the time it reached the STI clinic and was available to the patients and clinicians. The methodology of the audit has been depicted in the form of a flow chart in [Table/Fig-1], while the sample flow has been summarised in [Table/Fig-2].
Flow chart depicting the methodology of the audit
Flow chart depicting the sample flow
Results
A total of 200 consecutive VDRL requests from the STI clinic were analysed in this study. The majority of VDRL requests were for evaluation of lower abdominal pain (33%; 66 of 200) followed by vaginal discharge (26.5%; 53 of 200) and genital ulcer disease (9%; 18 of 200). A summary of the common indications for syphilis testing as observed in our study and of the demographic profile of the study population is given in [Table/Fig-3].
Demographic and clinical profile of study population (n=200)
| Variable | Number of cases (n=200) |
|---|
| Age (in years) | |
| Range | 16-60 |
| Mean | 31.42±8.46 |
| Median | 30 |
| Gender | |
| Male | 63 (31.5%) |
| Female | 137(68.5%) |
| Clinical presentation/diagnosis | |
| Lower abdominal pain | 66(33%) |
| Vaginal discharge | 53(26.5%) |
| Genital ulcer | 18(9%) |
| Genital warts | 13(6.5%) |
| Genital molluscum | 10(5%) |
| Chronic balanoposthitis | 9(4.5%) |
| Urethral discharge | 5(2.5%) |
| Suspected syphilis | 9(4.5%) |
| Pityriasis rosea | 1(0.5%) |
| Scabies | 4(2%) |
| Partner evaluation | 3(1.5%) |
| Burning micturition | 2(1%) |
| Not specified | 7(3.5%) |
The mean absolute turnaround time of VDRL test was 7.46±2.81 days (2-21 days) [Table/Fig-4]. On analysing the complete cycle of VDRL sample flow, we found that while the pre-laboratory phase is the shortest (mean duration=0 days), the laboratory phase comprises the longest time interval (mean duration=4.69±2.13 days).A breakup of the intradepartmental/laboratory turnaround time in days is given in [Table/Fig-5]. As can be seen from the table, the interval from specimen receipt to performance of tests (mean duration=4.25±1.96 days; range=1-10 days) accounts for nearly 90.6% of the duration of laboratory phase.
Description of the different phases of testing cycle (in days)
| Parameter | Pre-laboratory phase | Laboratory phase | Post-laboratory phase | Absolute turn-around time |
|---|
| Mean | 0 | 4.69±2.13 | 2.77±2.51 | 7.46±2.81 |
| Median | 0 | 5 | 2.5 | 7 |
| Range | 0 | 1-11 | 0-10 | 2-21 |
A breakup of the laboratory phase in days
| Timepoints | Mean | Median | Range |
|---|
| Specimen receipt to performance of test | 4.25±1.96 | 4 | 1-10 |
| Performance of tests to generation of reports | 0.44±0.57 | 0 | 0-2 |
| Total intradepartmental turnaround time | 4.69±2.13 | 5 | 1-11 |
Four (2%) of the 200 sera were reactive for non-treponemal antibodies by VDRL test. Of these four patients, two presented with genital molluscum, one with symptoms and signs suggestive of syphilis and one was advised VDRL testing as a part of partner evaluation. Since the confirmatory treponemal test (TPHA) was performed on the VDRL reactive samples on the same day, the turnaround time for positive VDRL test results did not differ from those for negative results.
Of the 200 patients tested, 58(29%) did not come for a subsequent visit to collect their VDRL test report. Thus the follow-up rate for VDRL testing among STI clinic attendees was 71% (142 of 200). While 142 (72.4%) of the 196 patients who were negative for VDRL test came for follow-up; none of the VDRL reactive patients came to collect their reports (follow-up rate 0%). Among the patients who came for a follow-up visit, the average time from physician request for VDRL test to report collection by the patient was 17.14±7.79 days (median=14 days; range=4-38 days).
Discussion
Of the various performance characteristics that define a laboratory’s service, timeliness or a faster turnaround time is of utmost importance to the clinicians, even more so than the precision and analytical quality of testing [7]. Different starting and terminal points have been used in different studies to define the testing cycle of any laboratory procedure. We have taken physician request to physician receipt of report as the time points since surveys have shown them to be the preferred start and end points respectively for the majority of physicians [8]. Also, keeping in view that non-analytical delays may be responsible for upto 96% of turnaround time, we realised that the definition of turnaround time be extended beyond the domain of intra-laboratory activities to the extra-laboratory phases of the testing cycle as well [9,10].
The present audit shows that the rate limiting step in the VDRL testing cycle is the analytical or the laboratory phase. Similar studies that have audited the turnaround of various other laboratory investigations have highlighted the shortage of highly trained technical personnel as the single most important cause of delay in the analytical phase [11]. Other reasons that have been cited are mainly technical such as difficulty with instruments, clerical delays, laboratory accidents etc. [11]. We did not come across technical reasons as a cause of delay except on one occasion when the control panel gave invalid results with the antigen emulsion and the entire batch of samples had to be run again the next day with freshly prepared antigen of a different lot. As far as the VDRL testing cycle is concerned, the reasons of delay in the laboratory phase are quite different. The single most important cause of delay in this phase is the frequency at which the test procedure is put up. The test is best suited for batch testing of samples and even in healthcare facilities like ours where the sample load for syphilis serology ranges beyond 50 samples per day, the test is generally put every second or third day, in order to strike a balance between optimal utilization of reagents and resources and the need for timely reporting of VDRL test results.
Before we suggest any corrective actions, we need to resolve an important issue. Why do we desire for faster turnaround time of laboratory tests? The answer would be to reach a diagnosis as early as possible. With this goal in mind, would it not be better for a healthcare facility undertaking syphilis serology, to shift from a VDRL based algorithm for syphilis serodiagnosis to a decentralized approach with the use of rapid point of care procedures such as Rapid plasma reagin (RPR) test or immunochromatographic assays. The use of point of care procedures would not only significantly reduce the laboratory phase, the on-site performance of the test would also cut down the time spent on specimen transport from the collection site to the laboratory and for report despatch from the laboratory to the physician. Another advantage of RPR is that it would allow for plasma analysis, that is much faster than serum testing. In addition, the approach of decentralized testing where the test report is available to the patient and the physician on the same visit would resolve the problem of low follow-up rates in STI clinics and allow prompt initiation of treatment for syphilis in the first visit itself.
In resource constrained settings like India, where VDRL test might prove to be more cost-effective than RPR or immunochromatographic assays, an alternative approach to attaining a shorter laboratory phase would be to increase the frequency at which the VDRL test is put up. However, several other extraneous factors drive the time duration of the testing cycle. Existence of undergraduate and/or postgraduate teaching responsibilities as in a tertiary care teaching facility like ours is an important factor [12]. This is well supported by other studies that have documented laboratory test results to be available sooner in non-teaching than teaching facilities [13].
While the rate limiting step in VDRL testing cycle is the laboratory phase, the significance of cutting down on pre and post-laboratory phases cannot be underestimated. With regard to pre-analytical phase, the only cause of delay is the time spent on specimen transport. Use of point of care procedures can resolve the problem. In the western world, installation of pneumatic tube systems to facilitate sample transport has proved an effective way to shorten this phase [14]. The post–laboratory phase, which is next only to the laboratory phase in time duration, can be shortened by computerisation of laboratory services with terminals in all clinical areas to facilitate easy access of reports [5,15,16]. In resource constrained settings such as India, telephonically conveying the results to the clinicians and bypassing the time spent on report preparation and despatch seems the next best alternative.
Our review of VDRL requisition forms revealed that patients attend STI clinics seeking care for a myriad of clinical presentations and are routinely recommended screening for syphilis by the clinicians regardless of their symptom profile. In the setting of STI clinic this approach is justified since the presence of one STI increases the risk of acquisition of others making it likely that a person may be suffering from multiple STIs at the same time, some of which may even be clinically silent. This strategy of routinely screening STI clinic attendees for syphilis would allow early diagnosis and treatment of the patients before they unknowingly and silently transmit the infection to others.
Our study has revealed low follow-up rates for VDRL report collection in STI clinics. Of particular concern is the astonishingly low follow-up rate (0%) among patients who were reactive by VDRL test. Poor return of patients associated with off-site syphilis screening has also been reported in other studies [17]. As mentioned before, for high risk clients like those attending STI clinics, test reports need to be conveyed to the patients and to the treating clinicians as early as possible for timely institution of appropriate therapy; and in case of syphilis, the first line treatment for which is generally not included in empirical coverage given to the patients at initial visit, losing a reactive patient to follow-up could be potentially disastrous. We strongly recommend the use of point of care procedures for syphilis diagnosis to overcome the problem of low follow-up rates in STI facilities.
Our audit, though a simple one, has provided useful information about the turnaround time of VDRL test and has also highlighted the lacunae that exist in the current VDRL based testing protocol for syphilis serology that is characterized by unusually long turnaround times and poor patient follow-up rates. This process mapping where we scrutinised each and every step has helped us to identify the rate limiting links in the VDRL based testing algorithm. Not only has the survey enabled a formal quantification of the turnaround time of our testing facility, but has also provided a baseline reference to benchmark and assess our future performance.
Conclusion
The approach of VDRL based testing for serodiagnosis of syphilis is fraught with certain limitations, such as long turnaround time and low patient follow-up rates. Thus, in order to circumvent these problems, there is a strong need to shift to a decentralized approach with the use of rapid point of care procedures. This would allow early diagnosis and prompt initiation of treatment for syphilis in the first patient visit itself.