The caries process is characterizd by demineralization of the inorganic portion and destruction of the organic substance of the hard dental tissues which often leads to cavitation. Any mechanism that inhibits such acid production, increases resistance to demineralization, and/or facilitates remineralization is of considerable clinical interest [1]. The benefits of fluoride in the reduction of dental caries have been known for years but its exact mechanism of action is not completely understood. The mechanism by which fluoride increases caries resistance may arise from both systemic and topical applications of fluoride. The addition of fluoride leads to the formation of fluoroapatite and fluorohydroxyapatite which are more resistant to acid dissolution [2]. The delivery systems for fluoride for the professional application include prophylactic pastes, mouthwash solutions and pit and fissure sealants, and for unsupervised home use include fluoride dentifrices and rinses [3]. The addition of fluoride to restorative materials has attracted the attention of dental researchers and clinicians as for the possibility of using these materials as a source of low fluoride release to the teeth. These so called fluoride releasing dental materials have been elaborated with the purpose of reducing secondary caries and neutralizing the pH decrease especially in high risk caries patients [3].
Conventional glass ionomers and resin modified glass ionomers dental restoratives release high amount of fluoride followed by compomers and composites which release low levels of fluoride and the amalgam which releases no fluoride [4]. Various factors which govern the fluoride release from restorative materials are their composition, powder liquid ratio, setting mechanisms and fluoride content, nature of fluoride incorporated into resin based materials and several environmental conditions [5]. But the more relevant question is “Can these beneficial effects be maintained?” Most of the fluoride release studies performed with fluoridated dental materials have evaluated the amount of fluoride released over varying lengths of time without the material being exposed to exogenous sources of fluoride. Exposure of fluoride containing dental materials to exogenous fluoride sources replenishes the fluoride within the dental material and serves as a reservoir for continued fluoride release [4]. The glass component of the material and the structure of the hydrogel layer around glass filler particles following reactions between the glass and polyacid component are the underlying mechanism for the fluoride re release after recharging [6]. The present study was therefore conducted with the purpose of evaluating the fluoride releasing ability of the Glass ionomer cements, Compomers and Giomers and the effectiveness of topical fluorides on the fluoride releasing ability.
Materials and Methods
Present in vitro study was conducted in the Department of Conservative Dentistry and Endodontics, Rama Dental College, Kanpur with the clearance from ethical committee. Four different materials G.C.FUJI II., G.C. FUJI II. LC, BEAUTIFIL II, DYRACT were tested in the present study and grouped into 4 categories containing 15 samples each [Table/Fig-1].
Restorative materials used in the study
| Group (N=15) | Type | Product | Manufacturer | Shade | Code |
|---|
| Group 1 | GIC | GC Fuji Π | GC Corporation Tokyo, Japan | A3 | FII |
| Group 2 | RMGIC | GC Fuji Π LC | GC Corporation Tokyo, Japan | A3 | FII LC |
| Group 3 | Giomer | Beautifil II | Shofu. | A3 | BT |
| Group 4 | Compomer | Dyract | Dentsply India Ltd. | A3 | D |
Preparation of the specimen
Round stainless steel moulds (5mm in diameter, 3mm in depth) were used to prepare the required samples. The materials were prepared according to the manufacturer’s instructions and packed into the moulds. A thin layer of Vaseline was used to coat lateral surfaces of the mould to prevent material adhesion. The unwaxed dental floss was held in the center of mould. The specimen top surface was covered by a mylar strip and glass slides, and allowed to set at room temperature for 10 seconds in chemically curing material. The light curing materials were cured from top and bottom using a light source (QHL 75 curing unit Dentsply, Germany) for 40 seconds. Before setting, the specimens in each individual group were incubated in a 95% relative humidity environment at 37°C for 24 hours. Then specimens of each group were immersed in 20 ml deionized water (Guru Kripa Industries, Kanpur, India) in plastic bottles and stored in the incubator at 37°C.
Fluoride release
After 24 hours, the containers were thoroughly shaken and then the samples removed, dried and returned into a new vial containing 20 ml of deionized water. The cumulative fluoride release measurement was made during 1st day, 7th day and 15th day.
Topical Fluoride exposure protocol: After 15 days of initial fluoride release the samples of each group were divided into: 3 Sub Groups of five samples each. Sub Group A - Control group – No topical fluoride application. Sub Group B - The samples were exposed to 2% neutral sodium fluoride solution for 4 minutes and washed with copious deionized water for 10 sec and dried on absorbent paper. Sub Group C - The samples were hand brushed with a fluoridated dentifrice (1000ppm) for four minutes and then wiped clean with a tissue and rinsed for 10 sec using copious deionized water and dried. Each sample after fluoride application was suspended in plastic bottles containing 20 ml of deionized water and incubated at 37°c for 24 hours.
Fluoride re-releases
Deionized water was then analysed for fluoride release on 1st, 7th and 15th day using ion specific electrode. After 24 hours samples were removed from the bottle, washed with 1ml of double distilled water using a syringe, dried on absorbent paper and then restored in 20 ml of fresh deionized water.
Measurement of fluoride release: For the analysis of fluoride released from materials into aqueous solutions, an ion-selective electrode (ISE) attached to an ion meter is most commonly used. A total ionic strength adjustment buffer (TISAB) solution is normally added to the solution in order to control pH and prevent to formation of fluoride complexes. The use of TISAB II frees fluoride ions bound to hydrogen and eliminates hydroxyl ion interference, so enabling an accurate measurement of the total fluoride content.
Statistical Analysis
Statistical analysis was performed using Analysis of Variance (ANOVA) for multiple groups and Tukeys multiple post hoc procedure (T HSD) for pair wise comparison of two groups.
Results
Analysis of data revealed that there was significant difference in fluoride release on different days and different materials and also in fluoride re- release before and after recharge between different days and materials (p<0.05).
Initial Fluoride Release
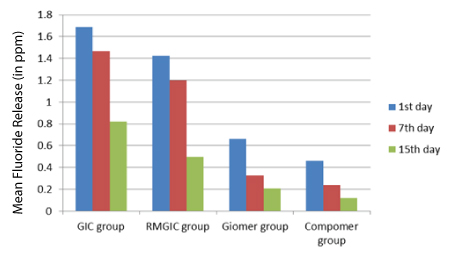
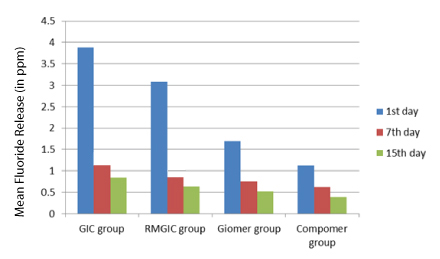
Comparing the four groups (GIC, RMGIC, Giomer, Compomer) the maximum cumulative initial fluoride release was related to GIC followed by RMGIC, Giomer, Compomer [Table/Fig-2]. On comparison of 1st, 7th and 15th day fluoride release, the fluoride release from all the materials was highest on 1st day decrease gradually till the 7th day but after that there was sharp decline in fluoride release from GIC and RMGIC where as for Giomer and Compomer values remained almost constant over time [Table/Fig-3].
Comparison of four groups (GIC group, RMGIC group, Giomer group, Compomer group) with respect to Fluoride release (in ppm) at 1st day, 7th day and 15th day
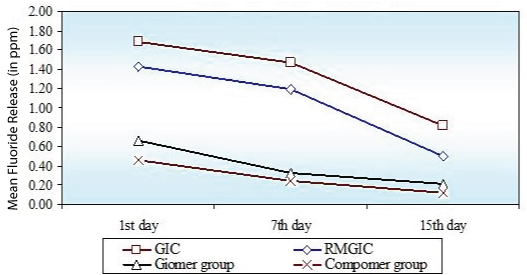
Comparison of 1st day, 7th day and 15th day with respect to fluoride release (in ppm) in four groups (GIC group, RMGIC group, Giomer group, Compomer group)
Fluoride Re Release
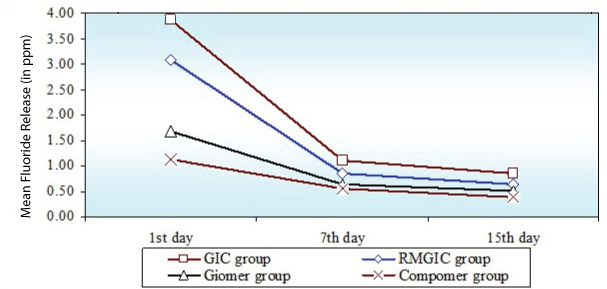
On recharging of different restorative materials the fluoride re release was high when 2%NaF solution (professional regime) [Table/Fig-4] was used as compared to fluoridated dentrifice (Home regime) [Table/Fig-5]. GIC had a greater recharge potential followed by RMGIC. Out of Giomer and Compomer, Giomer had a greater recharge potential. In both the regimes fluoride re release was greatest on the first day, decreased sharply till the 7th day after which the decrease was gradual [Table/Fig-6].
Comparison of four groups (GIC group, RMGIC group, Giomer group, Compomer group) with respect to Fluoride re release (in ppm) in Sub Group B at 1st day, 7th day and 15th day
Comparison of four groups (GIC group, RMGIC group, Giomer group, Compomer group) with respect to Fluoride re release (in ppm) in Sub Group C at 1st day, 7th day and 15th day
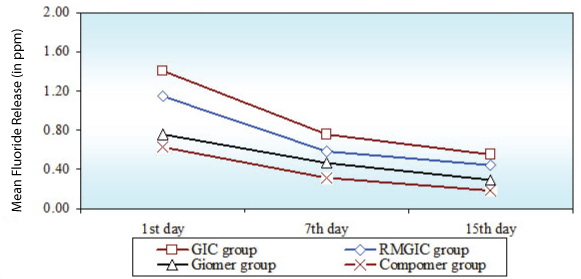
Comparison of 1st day, 7th day and 15th day with respect to fluoride re release (in ppm) in four groups (GIC group, RMGIC group, Giomer group, Compomer group) in Sub Group B
Discussion
Several studies have been conducted in the past to present time with considerably varying results of fluoride release from different dental materials [1,4,7]. Reasons for different results obtained can be attributed to different methodology and specimen size, storage media, frequency of change of storage media, quantity of media used to measure fluoride level [5].
The fluoride content and its release from restorative materials should be high without altering its physical properties and undue degradation of the filling. An initial high level of fluoride release in the vicinity of restoration will reduce the viability of bacteria thus inhibit dental caries by inducing remineralization of enamel/dentin [8].
On the 1st day the fluoride release is induced by superficial rinsing effect and during the subsequent days release is attributed to its ability to diffuse through cement pores and fractures [9].
This study evaluated the initial fluoride release from four materials over a period of fifteen days and fluoride re release after recharging with professional and home regime over a period of next fifteen days. In this study, deionized water was used as a specimen storage solution because it is easily obtainable and more fluoride is released in deionized water than in artificial saliva [10]. Therefore, the amount of fluoride released cannot be expected to be released from the specimens at the same content as occurred inside ones mouth.
The results of this current study agree with others that only GICs showed an initial fluoride burst effect [11]. The first process is characterized by an initial burst of fluoride release from the surface after which the elution is markedly reduced, accompanied by the second bulk diffusion process by which small amounts of fluoride continue to be released into the surrounding media. This pattern of release has been observed in previous studies [12]. In contrast to the Conventional and Resin modified glass ionomers, Giomers and Polyacid modified composites are shown to have no initial fluoride burst effect but levels of fluoride release remain relatively constant over time. Although the Giomer did not have an initial burst effect, its cumulative fluoride release was higher than Compomer [13].
On comparison of the amount of initial fluoride release, dental restoratives in descending order were conventional GIC, RMGIC, Giomer and Compomer. This is in accordance with the studies of Vermeersch, Leloup Vreven and Deniz C Can Karabulut et al. This order can be explained by the extent to which a glass ionomer matrix layer surrounds the glass filler in the set material [6]. For conventional GIC, after powder and liquid are mixed together, the ion leachable glass is decomposed by proton attack at the surface, and subsequently fluoride ions are liberated from the glass particles [11]. The acid base reaction is more extensive in conventional GIC and this result in a better defined matrix layer in these materials [6]. Initial setting of Resin modified glass ionomers is performed by light activated polymerization followed by an acid base reaction arises from sorption of water [5]. Both the type and amount of resin used for the photochemical polymerization reaction may affect the fluoride release from resin modified glass ionomers [7]. Giomers use pre reacted glass ionomer technology to form a stable phase of GIC in the restoration. The more extensive acid base reaction and hydrogel layer of glass fillers are responsible for high amount of release in giomers when compared to compomers [14].
Compomers contains a mixture of cycloaliphatic dicarboxylic acid dimethacryiate substitute for carboxylic acid and reactive glass fillers. It is claimed that the initially light polymerized material takes up water with time and that the carboxylic groups of the acidic monomer can undergo acid base reaction with metal ions of glass filler [15]. Fluoride release can occur in response to water uptake subsequent to dissolution of the glass filler particles or the ionic reaction on the surface of the glass particles [14]. Glass ionomer formulations can be recharged and release fluoride slowly after exposure to fluoride solutions such as toothpaste and fluoride rinses [11,15,16]. This may be clinically important because glass ionomer restorations may act as intraoral devices for the controlled slow release of fluoride at sites at risk for recurrent caries. Composites and Compomers, however, do not seem to have this ability [12,17]. Release of fluoride after topical application is dependent on the pH, concentration, dose, duration and frequency of application [14]. All the restorative materials could be recharged by both the agents used in the study but as seen in the study the recharge after 2 % NaF application was greater than toothpaste application suggesting professional topical fluorides could recharge the restoratives greater than home regime. Professional topical fluoride applications usually contain 2% NaF (9000ppm F) where as home regime; toothpaste contains (1000ppm F) [18]. Professionally applied fluoride treatment provides a 2.5 to 4 fold increase in fluoride release from fluoride containing dental materials [4]. Sodium fluoride solution induce disintegration of the matrix regions around glass fillers leading to increased ability of the fluoride recharge [6]. Our study confirms the findings of Takahashi et al., who demonstrated that fluoride release by glass ionomer materials increases with exposure to increased fluoride concentration [12]. In a study conducted by Freedman and Diefendefer, it was concluded that home care fluoride exposures provided adequate measurable fluoride uptake and subsequently release in these materials, with a higher level for resin modified glass ionomers [8].
Independent of time and the recharging agent used, when the total amount of fluoride re released after recharging was compared among the materials, Conventional GIC gave significantly greater amounts followed by RMGIC, Giomer and Compomer. This is probably because GIC has a well established glass ionomer matrix around the glass filler particles. This phase not only promotes fluoride release but also recharging [6]. This may allow deeper penetration of the fluoride recharging agent into materials having a substantial glass ionomer matrix component. Xu and Burgess suggested that material with higher fluoride release has a higher fluoride recharging ability [5].
After application of fluoride regimes the fluoride release from Conventional and Resin modified glass ionomer cements could be increased and prolonged [17]. The fluoride re release was less for Compomers and Giomers suggesting that recharging ability of the glass ionomer cements was superior to that of Giomers and Compomers. This fact may be explained by the loosely bound water and the solutes in the porosities in the glass ionomer, which could be exchanged with an external medium by passive diffusion [19]. Capability of the Giomers to recharging can be attributed to the well established glass ionomer matrix around glass filler particles.
Irrespective of the recharging agent used, all the restorative materials showed an increased amount of fluoride re release on recharging on the 1st day followed by rapid return to near exposure levels suggesting the fluoride release after exposure to topical fluoride represents only a washout of ions adsorbed to the surface, rather than an actual diffusion into the matrix [20].
Limitations of The Study
Within the limitations of the study design (specimen size, storage media, etc) definitive conclusion cannot be made and further in vivo investigations are needed to evaluate fluoride release after exposure to fluoride regime under the dynamic conditions of oral cavity. The clinical significance of the released fluoride is yet to be fully confirmed. Many factors such as the site into which the fluoride diffuses and the rate of diffusion will influence its anticaries effectiveness. The ultimate goal of correlating fluoride release with actual caries reduction is an objective that can only be met by completing controlled clinical studies on materials with well characterized kinetics of fluoride release.
Conclusion
The initial Fluoride release was highest from Conventional GIC followed by Resin Modified GIC, Giomer and Compomer. The Fluoride re release was high when recharging with professional regime (2% NaF) as compared to home regime (Toothpaste). Conventional GIC had a greater recharging ability followed by Resin Modified GIC, Giomer and Compomer.