Invitro Anti-mycotic Activity of Hydro Alcoholic Extracts of Some Indian Medicinal Plants against Fluconazole Resistant Candida albicans
Saranya Varadarajan1, Malathi Narasimhan2, Malaiyandi Malaisamy3, Chamundeeswari Duraipandian4
1 Senior Research Fellow, Department of Oral Pathology, Faculty of Dental sciences, Sri Ramachandra University, Chennai, India.
2 Professor and Head, Department of Oral Pathology, Faculty of Dental Sciences, Sri Ramachandra University, Chennai, India.
3 Research Scholar, Center for Advanced Studies in Botany, Guindy Campus, University of Madras, Chennai, India.
4 Principal, College of Pharmacy, Sri Ramachandra University.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Malathi Narasimhan, Professor and Head, Department of Oral Pathology, Faculty of Dental Sciences, Sri Ramachandra University, Porur, Chennai-600116, India.
E-mail: narasimhan.malathi@gmail.com
Background
Candidiasis is one of the most common opportunistic infections caused by Candida albicans. Fluconazole is the drug of choice for prevention and management of this condition. However, the emergence of fluconazole resistant candidal strains has become a major concern. Many herbs like fenugreek, cinnamon, papaya, oregano, garlic are rich in phytochemical constituents known to express antimycotic activity. With the available information, the present research study was carried out to assess the invitro anti-mycotic activity of hydro alcoholic extracts of Trigonella foenum-graecum seeds, Cinnamomum verum bark and Carica papaya leaves and seeds against fluconazole resistant Candida albicans
Materials and Methods
Hydro alcoholic extracts of Trigonella foenum-graecum (seeds), Cinnamomum verum (bark), Carica papaya CO.2 strain (male and female leaves) and Carica papaya CO.2 strain (seeds) were prepared by maceration. The anti-mycotic activity of the prepared extracts against Candida albicans was assessed by agar well diffusion method. Three independent experiments were performed in triplicates and the mean and standard deviation were calculated. Minimum inhibitory concentration was determined.
Results
The results of the present study revealed that all the extracts exhibited anti-mycotic activity in a dose dependent manner and minimum inhibitory concentration of all the extracts was found to be 15.62 μg/ml.
Conclusion
The results of the present study shed light on the fact that plant extracts could be used not only as an alternate drug for management of fluconazole resistant candidiasis but also explored further for oral cancer prevention as a therapeutic adjunct.
Carica papaya, Cinnamomum verum, Trigonella foenum graecum
Introduction
Candida albicans formerly known as Monilia albicans is a yeast like fungus that belongs to the family Sacharomycetaceae. The other names include Candida stellatoidea and Odium albicans. This fungus exhibits three forms viz- yeast, pesudohyphae, and chlamydospore. It occurs as a commensal in the oral cavity and in the gastrointestinal tract of humans. Candidiasis is one of the most common opportunistic infections caused by the organism. In the oral cavity, candidiasis is termed as oral thrush due to the formation of white scrapable pseudomembrane. Oral candidal infection occurs in immunosuppressed conditions like acquired immunodeficiency syndrome, cancer chemotherapy and head and neck radiotherapy [1,2]. Candida albicans has also been implicated in oral carcinogenesis. Candida albicans metabolizes procarcinogens like ethanol and forms acetaldehyde. It also causes nitrosamine production. It also alters tumour microenvironment and induces chronic inflammation [3].
Fluconazole has been used as a “Gold Standard” for management of candidiasis as it has been found effective in both immune compromised and immunocompetent individuals. It has also been used prophylactically to prevent infections in patients receiving chemotherapy and radiotherapy [4]. Fluconazole exerts its antifungal activity by inhibition of 14 alpha lanosteroldemethylase. This leads to accumulation of lanosterol and 14 alpha methylated sterols in the cell membrane of fungi that alters membrane permeability ultimately leading to fungal death [5].
The various mechanisms of fluconazole resistance are explained as follows:
Point mutations could occur in the ERG11 gene that codes for the enzyme lanosterol 14- alpha demethylase leading to reduced drug affinity to the enzyme product.
There could also occur an overexpression of the ERG 11 gene leading to the increased synthesis of ergosterol and other steroids that support fungal growth.
An overexpression of CDR gene (an ABC transporter) and MDR (a major faciltator) could also occur that causes reduction of fluconazole accumulation inside the fungal cell and reduced bioavailability of the same [6].
In this regard, herbs and naturally derived bioactive compounds have been explored for anti-mycotic therapy against resistant pathogens. Herbs are rich in phytochemical constituents like polyphenols, flavonoids, alkaloids, terpenoids, tannins, and glucosinolates that possess antioxidant, antimicrobial and immunomodulatory properties. Trigonella foenum graceum commonly called as fenugreek, Cinnamomum verum also called as Celyon cinnamon, Carica papaya commonly known as papaya possess phytochemicals that are known to exert antimicrobial activity. Moreover these herbs are a part of the normal Indian diet and can be procured in a cost effective manner. With the available information we set out to assess the anti-mycotic effect of hydro-alcoholic extracts of Trigonella foenum-graecum (seeds), Cinnamomum verum (bark) and Carica papaya (leaves and seeds) against fluconazole resistant Candida albicans.
Materials and Methods
The study was conducted in 2014 in Faculty of Dental Sciences and Faculty of Pharmacy, Sri Ramachandra University. This study has an invitro design.
Collection of plant material and preparation of extracts was done as previously described [7].
Plant material:Trigonella foenum-graecum (seeds) Cinnamomum verum (bark) were collected from a reputed organic store in Chennai and reputed spice market in Coimbatore respectively. Carica papaya CO.2 strain (male and female leaves) and Carica papaya CO.2 strain (seeds) were collected from Tamil Nadu Agricultural University Coimbatore. All the herbs were authenticated by Professor P. Jayaraman, Plant Anatomy Research Center, Tamil Nadu, Chennai, India.
Preparation of extracts: Preparation of extracts and fluconazole: Hydroalcoholic extracts of Trigonella foenum-graecum (seeds) (60 ethanol: 40 water; v/v), Cinnamomum verum bark (70 ethanol: 30 water; v/v), Carica papaya CO.2 strain (male and female leaves) (60 ethanol: 40 water; v/v) and Carica papaya CO.2 strain (seeds) (60 ethanol: 40 water; v/v) were prepared by maceration for 72, 48 and 24 hours. All the extracts individually were pooled together and concentrated using rotary flash followed by vacuum desiccator and stored at 2-4°C until use. Stock solution of the extracts was prepared by dissolving the extract in Dimethylsulfoxide (DMSO). Serial dilutions were done to obtain concentrations of 250, 500, 1000 μg/mL. Fluconazole was procured from Sigma Aldrich and a working concentration of 30 μg/mL was prepared in sterile distilled water.
Preparation of Candia albicans Culture:Candida albicans MTTC 227 was procured from Microbial Type Culture Collection and Gene Bank, Chandigarh. The Ampule was thawed in a water bath at 25°C for 2 minutes, which was wiped with 70% ethanol and was transferred to potato dextrose agar and incubated at 28°C. Drug resistance was induced according to the protocol of Yan L et al., with modifications [8]. Briefly a colony of Candida albicans culture was inoculated into potato dextrose broth and incubated overnight at 30°C in an orbit shaker at 200 rpm (revolutions per minute). An aliquot of this culture containing 106 cells was treated with twice the concentration of recently measured minimum inhibitory concentration of fluconazole and incubated 30°C at in orbit shaker at 200 rpm. When the cultures attained a density of 108 cells an aliquot of 106 cells was taken and the procedure was repeated. Antibiotic sensitivity test for the last passage was performed to confirm fluconazole resistance and the strain was used for the study. Colonies of resistant strain of C. albicans were inoculated in potato dextrose broth incubated at 37°C for 24 hours in an orbit shaker at 200 rpm. Standard inoculum of the fungus of 1.5×106 colony forming units (CFU mL-1) was diluted to 1:100 and turbidity was adjusted to match a McFarland standard [9].
Antimycotic activity:Candida albicans was swabbed onto potato dextrose agar using sterile swab sticks. Wells of 9 mm diameter were cut using sterile cork borer. The fungal cultures were treated with hydro alcoholic extracts of T. foenum-graecum (seeds), C. verum (bark), C. papaya CO.2 strain (male and female leaves and seeds) at different concentrations 250, 500, 1000 μg/mL using sterile microtips. Drug loaded plates were incubated at 37°C for 24 hour. The zone of clearance was measured. Three independent experiments were performed in triplicates and mean and standard deviation was calculated.
The percentage inhibition was calculated using the formula [10].

Determination of Minimum inhibitory concentration: Hydroalcoholic extracts which showed significant zones of inhibition were chosen for the experiment at concentrations of 500 μg/ml, 250 μg/ml, 125 μg/ml, 62.5 μg/ml, 31.25 μg/ml, 15.62 μg/ml and 7.81μg/ml. Minimum Inhibitory Concentration (MIC) was determined according to the standard protocol of Wariso and Ebong (1996) with slight modifications [11]. The antimycotic activity was classified based on MIC [12,13] as follows:
<100 μg/ml – good
100-500 μg/ml – moderate
500-1000 μg/ml – weak
>1000 μg/ml – inactive
Results
All the extracts exhibited antimycotic activity in a dose dependent manner with zone of inhibition ranging between 10±0.7 to 26±1.82 μg/ml. Minimum Inhibitory concentration of all the extracts was found to be15.62 μg/ml. The results are depicted in [Table/Fig-1,2, and 3].
Antifungal of hydroalcoholic extracts of Trigonellafoenum-graecum(seeds), Cinnamomumverum(bark) and Carica papaya (leaves and seeds) against fluconazole resistant Candida albicans. Values are mean ± standard deviation of triplicates of three independent experiments
| Samples | Organisms | Zone of inhibition in mm | Percentage of Inhibition (%) |
|---|
| 250 μg | 500 μg | 1000 μg | 250 μg | 500 μg | 1000 μg |
|---|
| Trigonella foenum graceum (seeds) | Candida albicans | 10±0.7 | 12±0.84 | 14±0.98 | 11.11±0.78 | 13.33±0.93 | 15.56±1.08 |
| Cinnamomum verum (bark) | 16±1.12 | 18±1.26 | 20±1.4 | 17.77±1.24 | 20.00±1.4 | 22.22±1.55 |
| Carica papaya (male leaves) | - | - | 15±1.05 | - | - | 16.67±1.16 |
| Carica papaya (female leaves) | 11±0.77 | 13±0.91 | 15±1.05 | 12.22±0.85 | 14.44±1.01 | 16.67±1.16 |
| Carica papaya (seeds) | 22±1.54 | 24±1.68 | 26±1.82 | 24.44±1.71 | 26.67±1.86 | 28.89±2.02 |
Minimum inhibitory concentration of selected medicinal plants
| Samples | MIC μg/mL |
|---|
| Trigonell afoenum-graceum (seeds) | 15.62 |
| Cinnamomum verum (bark) | 15.62 |
| Carica papaya (male leaves) | 15.62 |
| Carica papaya (female leaves) | 15.62 |
| Carica papaya (seeds) | 15.62 |
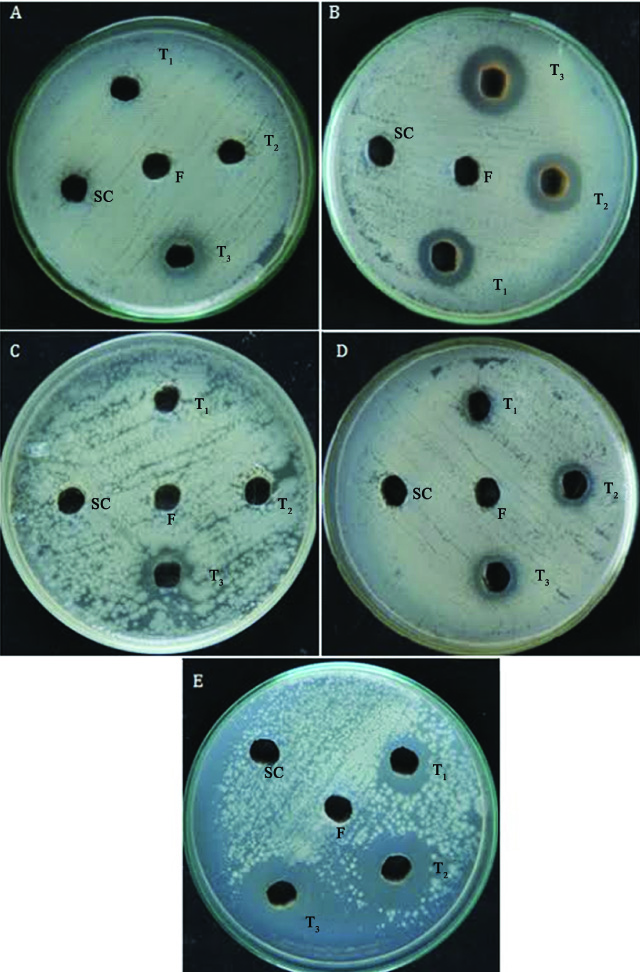
Antifungal of hydroalcoholic extracts of Trigonella foenum-graecum (seeds), Cinnamomum verum (bark) and Carica papaya (leaves and seeds) against fluconazole resistant Candida albicans.
a- Trigonellafoenum-graecum (seeds).
b- Cinnamomum verum (bark).
c- CO.2 strain Carica papaya (male leaves).
d- CO.2 strain Carica papaya (female leaves).
e- CO.2 strain Carica papaya (seeds).
f- Fluconazole, SC- solvent control, T1- 250 μg/ml, T2 500 μg/ml, T3- 1000 μg/ml
Discussion
Candida albicans a commensal of human oral and gastrointestinal flora and causes oral and systemic candidiasis in immunocompromised individuals, patients receiving chemotherapy and radiotherapy. It has also been implicated in oral carcinogenesis [3]. Fluconazole is one of the most common drugs used for prophylaxis as well as management of candidiasis as it has been found effective irrespective of the immune status of the individual receiving therapy. The emergence of fluconazole resistant strains warrants the need for alternate drugs.
Herbs and plant derived compounds have been used for the management of various diseases several hundred years ago by Charaka and Susrutha. Herbs and plants are rich in phytochemicals such as polyphenols, flavonoids, alkaloids that could exert antimycotic activity [14]. The antimicrobial and anti-mycotic activity of Trigonella foenum graecum (seeds), Cinnamomum verum (bark), Carica papaya (leaves and seeds) against different organisms has been demonstrated by various authors, however anti-mycotic activity against fluconazole resistant species has not been explored. With the available information we screened the invitro anti-mycotic activity of Trigoenlla foenum graecum (seeds), Cinnamomum verum (bark), Carica papaya (leaves and seeds) against fluconazole resistant Cancida albicans. The results of the present study revealed that all the extracts exhibited anti-mycotic activity in a dose dependent manner. The minimum inhibitory concentration of all the extracts depicts that all the extracts possesses good antimycotic activity [12,13].
The bioactive toxic oils, volatile oils and alkaloids of Trigonella foenum graecum (seeds) exert toxic effects on bacteria, parasites and fungi [15]. The anti-mycotic activity of Trigonella foenum graecum (seeds) could also be attributed to polyphenols and flavonoids, however the exact mechanism remains unclear. Faten Omezzine et al., have reported the anti-mycotic activity of Trigonella foenum graecum at various stages of development and levels of ploidy and have shown that five novel compounds kaempferol 7-O-glucoside, kaempferol 3-O-β-d-glucopyranoside, kaempferol 7-O-β-d-glucopyranosyl (1–4) β-d-glucopyranoside, kaempferol 3-O-α-l-rhamnosyl (1→2) β-d-xyloside, vitexin hexoside and kaempferol 3-O-β-glucosyl (1→2) (6′-O-acetyl)-β-d-galactoside property of the herb [16].
The anti-mycotic property of Cinnamomum verum could be attributed to the presence of Cinnamaldehyde which is one of the most important phytochemical constituent of the plant. The anti-mycotic activity of Cinnamaldehyde has been reported by various authors. Cinnamaldehyde irreversibly affects sterol biosynthesis and ATPase activity in plasma membrane of fungal cell. This leads to accumulation of H+ ions that alter cellular pH leading to cell death [17–21].
With regard to Carcia papaya (leaves), our results are concurrent with the findings of Nwachukwu et al., Pedro Chávez-Quintal et al.,. The terpenes, alkaloids and flavonoids of Carica papaya leaves could exert fungicidal effects by interaction with cell membrane of fungi, however the compounds responsible for the property have not been studied [22,23].
In the present study, Carica papaya (seeds) exhibited significant antimycotic activity. Onkar Singh et al., have reported the anti-mycotic activity of Carica papaya seeds and 2,3,4-trihydroxytoluene, a compound has been isolated from Carica papaya seeds with activity against Candida albicans [24]. Benzylisothiocyanate is another important phytochemical constituent in Carica papaya seeds that could exert antibacterial and fungicidal effects. Isothiocyanates form thiocarbazones and thioureas by reacting with thiol and amino group respectively which causes inhibition of proteins and enzymes essential for survival of bacterial and fungal cell. Derivatives of isothiocyantes also exert antimycotic property [25–27].
Limitation of The Study
It has an invitro design and clinical trials and toxicity studies have not been carried out.
Conclusion
The results of the present study shed light on the fact that plant extracts could be used not only as an alternate drug for management of fluconazole resistant candidiasis but also explored further for oral cancer prevention and therapeutic adjunct. Further studies have to be carried out to isolate the active compound and exact mechanism of fungicidal activity that would aid in development of newer drugs.
[1]. Lalla RV, Latortue MC, Hong CH, A systematic review of oral fungal infections in patients receiving cancer therapySupport Care Cancer 2010 18(8):985-92. [Google Scholar]
[2]. Sangeorzan JA, Bradley SF, He X, Zarins LT, Ridenour GL, Tiballi RN, Kauffman CA, Epidemiology of oral candidiasis in HIV-infected patients: Colonization, infection, treatment, and emergence of fluconazole resistanceThe American Journal of Medicine 1994 97(4):339-46. [Google Scholar]
[3]. Mohd Bakri M, Mohd Hussaini H, Rachel Holmes A, David Cannon R, Mary Rich A, Revisiting the association between candidal infection and carcinoma, particularly oral squamous cell carcinomaJournal of Oral Microbiology 2010 2:103402/jom.v2i0.5780 [Google Scholar]
[4]. Martin MV, The use of fluconazole and itraconazole in the treatment of Candida albicans infections: a reviewJournal of Antimicrobial Chemotherapy 1999 44:429-37. [Google Scholar]
[5]. Ghannoum MA, Rice LB, Antifungal Agents: Mode of Action, Mechanisms of Resistance, and Correlation of These with Bacterial ResistanceClin Microbiol Rev 1999 12(4):501-17. [Google Scholar]
[6]. Casalinuovo IA, Di Francesco P, Garaci E, Fluconazole resistance in Candida albicans: a review of mechanismsEuropean Review for Medical and Pharmacological Sciences 2004 8:69-77. [Google Scholar]
[7]. Saranya V, Malathi N, Chamundeeswari D, Sakthisekaran D, Invitro Antioxidant activities of hydro alcoholic extracts of Trigonella foenum-graecum seeds, Cinnamomum verum bark and Carica papaya leaves and seedsIndian Journal of Research in Pharmacy and Biotechnology 2014 2(6):1529-36. [Google Scholar]
[8]. Yan L, Zhang J, Li M, Cao Y, Xu Z, Cao Y, DNA microarray analysis of fluconazole resistance in a laboratory Candida albicans strainActa Biochim Biophys Sin (Shanghai) 2008 40(12):1048-60. [Google Scholar]
[9]. Sutton S, Determination of Innoculum for Microbiology TestingJ GXP Compliance 2011 15(3):49-53. [Google Scholar]
[10]. Perez C, Pauli M, Bazerque P, An antibiotic assay by the agar well diffusion methodActa Biol Med Exp 1990 15:113-15. [Google Scholar]
[11]. Wariso BA, Ebong O, Antimicrobial activity of kalanchoe pinnaata (Ntiele. Lam) persW Afr J Pharm Drug Res 1996 12:65-68. [Google Scholar]
[12]. Saez FJ, Variability in essential oil from populations of Thymus hyemalis Lange in southeastern SpainJ Herbs, Spices & Med Plants 1998 5:65-76. [Google Scholar]
[13]. Kucukbay FZ, Kuyumcu E, Celen S, Azaz AD, Arabac T, Chemical Composition of the Essential Oils of Three Thymus Taxa from Turkey with Antimicrobial and Antioxidant ActivitiesRec Nat Prod 2014 8(2):110-20. [Google Scholar]
[14]. Cowan MM, Plant Products as Antimicrobial AgentsClin Microbiol Rev 1999 12(4):564-82. [Google Scholar]
[15]. Kor NM, Didarshetaban MB, Pour SHR, Fenugreek (Trigonella foenum-graecum L.) As a Valuable Medicinal PlantInternational Journal of Advanced Biological and Biomedical Research 2013 1(8):922-31. [Google Scholar]
[16]. Omezzine F, Bouaziz M, Remadi MD, Simmonds Monique SJ, Haouala R, Chemical composition and antifungal activity of Trigonella foenum-graecum L. varied with plant ploidy level and developmental stageArabian Journal of ChemistryAvailable online 13 April 2013 [Google Scholar]
[17]. Ferhout H, Bohatier J, Guillot J, Chalcha JC, Antifungal Activity of Selected Essential Oils, Cinnamaldehyde and Carvacrol against Malassezia furfur and Candida albicansJournal of Essential Oil Research 1999 11(1):119-29. [Google Scholar]
[18]. Taguchi Y, Hasumi Y, Hayama K, Arai R, Nishiyama Y, Abe S, Effect of Cinnamaldehyde on Hyphal Growth of C. albicans Under Various Treatment ConditionsMed Mycol J Med Mycol J 2012 52(3):199-204. [Google Scholar]
[19]. Shreaz S, Bhatia R, Khan N, Muralidhar S, Basir SF, Manzoor N, Spice oil cinnamaldehyde exhibits potent anticandidal activity against fluconazole resistant clinical isolatesFitoterapia 2011 82(7):1012-20. [Google Scholar]
[20]. Shreaz S, Bhatia R, Khan N, Muralidhar S, Manzoor N, Khan LA, Influences of cinnamic aldehydes on H+ extrusion activity and ultra structure of CandidaJournal of Medical Microbiology 2013 62(2):232-40. [Google Scholar]
[21]. Jantan IB, Moharam BA K, Santhanam J, Jamal JA, Correlation Between Chemical Composition and Antifungal Activity of the Essential Oils of Eight Cinnamomum SpeciesPharmaceutical Biology 2008 46(6):406-12. [Google Scholar]
[22]. Nwachukwu EO, Umechuruba CI, Antifungal Activities of Some Leaf Extracts on Seed-borne Fungi of African Yam Bean Seeds, Seed Germination and Seedling EmergenceJ Appl Sci Environ Mgt 2001 5(1):29-32. [Google Scholar]
[23]. Quintal PC, Flores TG, Buenfil IR, Tintoré SG, Antifungal Activity in Ethanolic Extracts of Carica papaya L. cv. Maradol Leaves and SeedsIndian J Microbiol 2011 51(1):54-60. [Google Scholar]
[24]. Singh O, Ali M, Phytochemical and Antifungal Profiles of the Seeds of Carica Papaya LIndian J Pharm Sci 2011 73(4):447-51. [Google Scholar]
[25]. Adebiyi A, Adaikan PG, Modulation of jejunal contractions by extract of Carica papaya L. seedsPhytother Res 2005 19(7):628-32. [Google Scholar]
[26]. Aires A, Mota V.R, Saavedra M.J, Rosa E.A.S, Bennett R.N, The antimicrobial effects of glucosinolates and their respective enzymatic hydrolysis products on bacteria isolated from the human intestinal tract A. The Society for Applied MicrobiologyJournal of Applied Microbiology 2009 106:2086-95. [Google Scholar]
[27]. Drobnica L, Zemanova M, Nemec P, Antos K, Kristian P, Stullerova A, Antifungal activity of Isothiocyanates and Related CompoundsApplied Microbiology 1967 15(4):701-09. [Google Scholar]