A Successful Strategy for Surgical Treatment of Boerhaave’s Syndrome Sans Thoracotomy
Devbrata Radhikamohan Adhikari1, Ajeet Ramamani Tiwari2, Siddharth Vankipuram3, Aniruddha Prabhakar Chaphekar4, Ritesh Satardey5
1Assistant Professor, Department of General Surgery, Topiwala National Medical College and Bai Yamunabai Laxman Charitable Nair Hospital, Mumbai Central, India.
2Resident, Department of General Surgery, Topiwala National Medical College and Bai Yamunabai Laxman Charitable Nair Hospital, Mumbai Central, India.
3Resident, Department of General Surgery, Topiwala National Medical College and Bai Yamunabai Laxman Charitable Nair Hospital, Mumbai Central, India.
4Associate Professor, Department of General Surgery, Topiwala National Medical College and Bai Yamunabai Laxman Charitable Nair Hospital, Mumbai Central, India.
5Resident, Department of General Surgery, Topiwala National Medical College and Bai Yamunabai Laxman Charitable Nair Hospital, Mumbai Central, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Devbrata Radhikamohan Adhikari, Assistant Professor, Department of General Surgery, Topiwala National Medical College and Bai Yamunabai Laxman Charitable Nair Hospital, Mumbai Central-400008, India.
E-mail: docdev84@yahoo.com
Boerhaave’s syndrome is a rare life threatening condition that is often misdiagnosed and fatal if not treated promptly. While the gold standard is early surgical intervention, recent studies have showed success with conservative management. We report a case of Boerhaave’s syndrome that was managed conservatively by decompressive gastrostomy, feeding jejunostomy, bilateral intercostal drainage tubes with added proximal diverting cervical esophagostomy. The patient recovered completely and stoma closure was done two months later.
Conservative management, Esophagostomy, Intercostal drainage
Case Report
A 50-year-old male presented to the emergency room with severe epigastric pain radiating to chest following two bouts of coffee colored vomitus. He also gave a history of breathlessness associated with subcutaneous emphysema over neck and chest. There was no history of chest trauma. The patient was a chronic alcoholic since thirty years and consumed a large quantity of alcohol two hours before the onset of vomiting. He had pulmonary tuberculosis in the past for which he completed six months of anti-tubercular therapy. On examination, the patient was afebrile, tachypnoeic with pulse of 120/min and blood pressure of 150/100 mm Hg. Per abdominal examination revealed tenderness in the epigastrium without any signs of peritonitis. Chest examination demonstrated massive subcutaneous emphysema, decreased breath sounds in bilateral lung fields and positive Hamman’s sign.
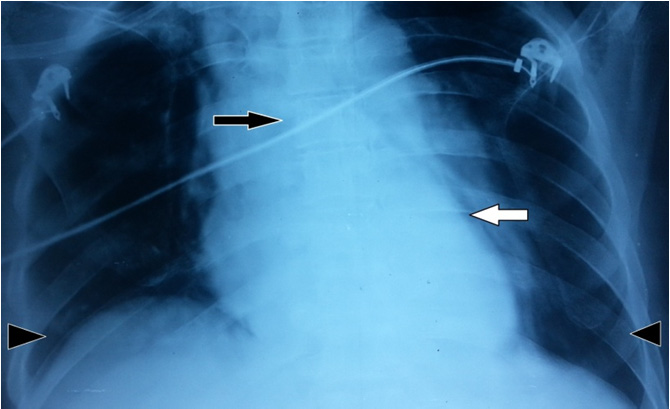
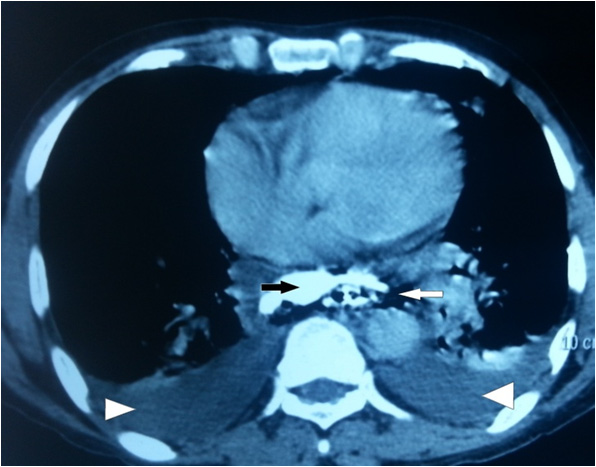
On admission, haemogram was normal with WBC counts of 6.4x103 /μl. BS was clinically diagnosed and X-ray of the chest showed mediastinal widening, pneumo-mediastinum and bilateral pleural effusion which confirmed the diagnosis of Boerhaave Syndrome [Table/Fig-1,2]. A CT scan of the thorax and abdomen revealed a leak of oral contrast and air from the right postero-lateral aspect of the lower one third of the esophagus with extension into the mediastinum and retroperitoneum [Table/Fig-3].
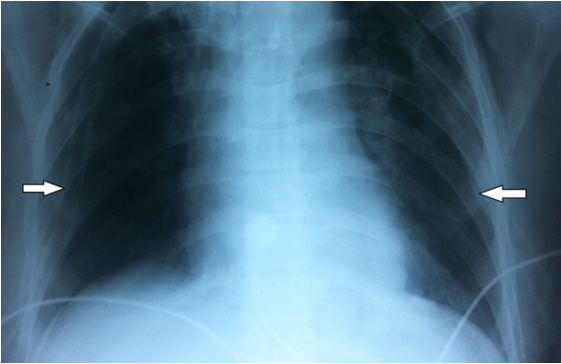
After resuscitating the patient, bilateral ICD’s were inserted which immediately drained 400 ml on the right side and 600 ml on the left side of dark brown fluid with food particles. The patient was then taken up for surgery (18 hours after the initial onset of symptoms). Exploratory laparotomy with decompressive gastrostomy and feeding jejunostomy was performed. A neck incision was made on the left side and a proximal diverting cervical esophagostomy was done. The patient remained in the ICU for two days and was then shifted to the ward. The ICD’s were draining 100-150 ml per day of dark brown fluid till day five of surgery and then gradually became negligible. Enteral feeds were started from the jejunostomy tube on day 3 of surgery and were gradually stepped up according to the patient’s BMI. A repeat CT esophagogram done six weeks later revealed no contrast extravasation. The patient was continued on conservative management for two months after which esophagostomy closure was done in single layer interrupted 3-0 PDS over a nasogastric tube. Patient withstood the procedure well and was discharged 71 days after admission. Patient was asymptomatic at the last follow up.
Discussion
Boerhaave’s syndrome is rare and carries a high mortality of upto 20-40% [1,2]. The best outcomes are achieved if surgical repair is performed within 24 hours of perforation [3]. Often, diagnosis and referral to specialized centres is delayed due to absence of physical signs, non-specific X-ray findings and low index of suspicion [4]. The cardinal symptoms are sudden lower thoracic pain associated with vomiting and subcutaneous emphysema [2,4]. The time between onset and diagnosis has definite therapeutic and prognostic implications [2,3].The differential diagnosis of Boerhaave’s syndrome includes a variety of thoracic and abdominal conditions including pulmonary embolism, dissecting aorta, ruptured aortic aneurysm, pancreatitis and perforated peptic ulcer [5].
The initial imaging procedure of choice is the plain X-ray of thorax and abdomen which rarely elicits any positive findings. The X-ray findings of our patient revealed mediastinal widening, pneumomediastinum and bilateral pleural effusion which was suggestive of BS. A CT scan of the thorax and abdomen confirmed the diagnosis and localized the site of perforation to the lower one-third thoracicesophagus [6].
Once a decision is taken to operate the patient, there are a variety of surgical options. Three factors determine the choice of treatment: the time (delay) of the diagnosis, the presence of septic complications, and the physiological reserves of the patient. In the indexed patient, although there was no delay in diagnosis and no evidence of sepsis, he was a chronic alcoholic with a past history of pulmonary tuberculosis. Taking these factors into consideration, we decided against a formal thoracotomy.
Though primary repair in such cases with early presentation is recommended, Vogel et al., demonstrated that conservative management without a thoractomy allows the native esophagus to heal [7]. We followed a similar conservative management with added cervical esophagostomy in view of mediastinal contamination. Biancari et al., performed a meta-analysis of 75 studies of all types of esophageal perforations and concluded that management is guided by the individual surgeon’s choice and expertise as there was a lack of data supporting the superiority of any one approach over the other [8].
There is little literature on the timing of the second CT esophagogram to confirm the patency of the healed perforation. Wu et al., presented an algorithm in their paper performing contrast CT esophagogram after 7-10 days of conservative treatment [9]. We performed the CT esophagogram 6 weeks after initial presentation.
Supine plain X-ray chest showing mediastinal widening (black arrow) with pneumomediastinum( white arrow) and bilateral pleural effusion (black arrow heads)
Upright plain X-ray chest demonstrating bilateral ICD in situ with resolving effusion (white arrows)
CT thorax with oral contrast showing leak in the right postero-lateral aspect of distal esophagus extending into the mediastinum (black arrow) with pneumo-mediastinum (white arrow) and bilateral pleural effusion (white arrowheads)
Conclusion
The above case highlights the advantages of avoiding a thoracotomy in terms of morbidity and associated chest complications. At the same time it may be prudent to accept the increased hospital stay associated with this line of management. Successful treatment of BS is a challenge to surgeons. Our patient benefitted from a cervical esophagostomy and conservative management which may hint that a select sub-group of cases of BS can be managed along similar lines avoiding the morbidity of a thoracotomy.
[1]. J Salo, E Sihvo, J Kauppi, J Räsänen, Boerhaave’s syndrome: lessons learned from 83 cases over three decadesScand J Surg 2013 102(4):271-73. [Google Scholar]
[2]. JA Søreide, A Viste, Esophageal perforation: Diagnostic work-up and clinical decision-making in the first 24 hoursScand J Trauma Resusc Emerg Med 2011 :19-66. [Google Scholar]
[3]. H Shaker, H Elsayed, I Whittle, S Hussein, M Shackcloth, The influence of the ‘golden 24-h rule’ on the prognosis of oesophageal perforation in the modern era.Eur J Cardiothorac Surg 2010 38:216-22. [Google Scholar]
[4]. M Chirica, A Champault, X Dray, L Sulpice, N Munoz-Bongrand, E Sarfati, Esophageal perforationsJ Visc Surg 2010 147(3):e117-28. [Google Scholar]
[5]. J Spapen, J De Regt, K Nieboer, G Verfaillie, PM Honoré, H Spapen, Boerhaave’s syndrome: still a diagnostic and therapeutic challenge in the 21st centuryCase Rep Crit Care 2013 :2013-161286. [Google Scholar]
[6]. CA Young, CO Menias, S Bhalla, SR Prasad, CT features of esophageal emergencies.Radiographics. 2008 28:1541-53. [Google Scholar]
[7]. SB Vogel, WR Rout, TD Martin, PL Abbitt, Esophageal perforation in adults: aggressive, conservative treatment lowers morbidity and mortalityAnn Surg 2005 241(6):1016-23. [Google Scholar]
[8]. F Biancari, V D’Andrea, R Paone, C Di Marco, G Savino, V Koivukangas, Current treatment and outcome of esophageal perforations in adults: systematic review and meta-analysis of 75 studiesWorld J Surg 2013 37:1051-59. [Google Scholar]
[9]. JT Wu, KL Mattox, MJ Wall, Esophageal perforations: new perspectives and treatmentJ Trauma 2007 63:1173-84. [Google Scholar]