Stevens Johnson Syndrome-Toxic Epidermal Necrolysis Overlap Secondary to Interaction Between Methotrexate and Etoricoxib: A Case Report
P.R. Rachana1, HV Anuradha2, Reddy Mounika3
1Post Graduate, Department of Pharmaology, M.S Ramaiah Medical College, Bangalore, India.
2Associate Professor, Department of Pharmacology, M.S Ramaiah Medical College, Bangalore, India.
3Post Graduate, Department of Dermatology, M.S Ramaiah Medical College, Bangalore, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. P.R Rachana, Post Graduate, Pharmaology, M.S Ramaiah Medical College, Bangalore, India.E-mail :rachana2287@gmail.com
Rheumatoid arthritis (RA) is an autoimmune disease affecting about 1% of people, with the highest incidence between 40 and 70 years. Methotrexate is an anti-folate analogue that has good efficacy and safety in the treatment of RA. Methotrexate (MTX) and non-steroidal anti inflammatory drugs are often concomitantly administered in clinical practice for the treatment of RA. In this case report, a 57-year-old female was treated with oral methotrexate 7.5 mg per week for a diagnosed case of RA. Since her pain persisted after completing six weeks of treatment with methotrexate, oral etoricoxib 60 mg once daily was added to the treatment regimen. Six weeks later, the patient complained of oral ulcerations and blisters on all fours limbs and trunk. The patient was re-evaluated and was diagnosed with Stevens-Johnson syndrome-Toxic epidermal necrolysis (SJS-TEN) overlap. This case highlights the possible pharmacokinetic interaction between methotrexate and etoricoxib that has a significant clinical implication.
Drug interaction, Non-steroidal anti-inflammatory drugs, Rheumatoid arthritis
Case Report
A 57-year-old female presented in Emergency Department of M.S Ramaiah Medical college, Bangalore with complaints of fever, loose stools, malaise, swelling of lips, oral ulcers and blisters all over the body. The patient had noticed the blisters a few days after developing fever. The patient had been diagnosed with rheumatoid arthritis three years ago and had been taking oral methotrexate 7.5 mg per week for six weeks.
Since her joint pain persisted, her family physician added oral etoricoxib 60 mg once daily to the treatment regimen. Patient continued to take etoricoxib 60mg daily for six weeks along with methotrexate. Other concomitant medications included amlodipine 2.5mg, atorvastatin 10mg, chloroquine 200 mg, omeprazole 20 mg and folic acid 5mg . No past history of allergic diathesis was reported.
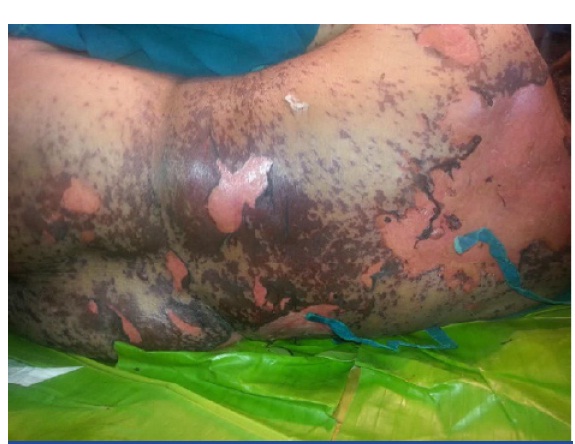
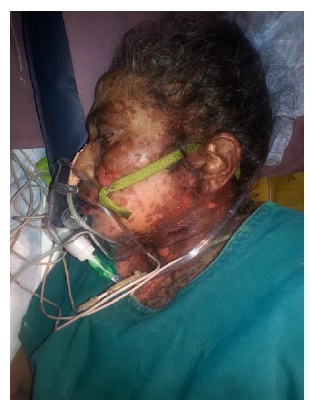
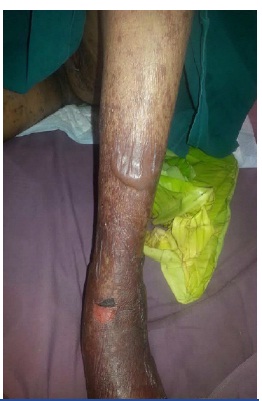
The physical examination was remarkable for cutaneous involvement of trunk, face, lips, palms and soles. Large areas of epidermal detachment involing more than 20% of body surface are were noticed [Table/Fig-1,2]. Bullous lesions with erosions and peeling were present bilaterally on both the legs [Table/Fig-3]. Oral erosions were seen. Conjunctival congestion was present. The vital signs recorded were as follows: temperature (axillary)-38.5ºC; pulse rate- 88/min; respiration rate- 18/min; blood pressure-160/80mmHg.
On admission laboratory investigations revealed haemoglobin 9.04 g/dl white blood cell count 5620 mm3, platelet count 160,000 mm3, serum creatinine 1.1 mg/dl, blood urea nitrogen 18 mg/dl, liver function test was normal. Pus culture was positive for gram positive cocci and coagulase negative Staphylococcus. Blood and urine cultures were sterile. Chest X- ray and ultrasound abdomen were normal.
After the patient’s initial assessment in the emergency room, consultations by a dermatologist and an ophthalmologist were availed. The diagnosis of SJS-TEN overlap was concurred. All the previous medications taken by the patient were discontinued at this time. The patient was shifted to the intensive care unit and her treatment involved fluid-electrolyte replacement; steroids (betamethasone 6 mg/day); a prophylactic antibiotic and antifungal (meropenem 1g, fluconazole 200 mg) due to the risk of Staphylococcus aureus and other opportunistic skin infections and sepsis; mouth care (nystatin oral suspension and lignocaine viscous fluid), topical wound care (fusidic acid and betamethasone acetate cream); eye care (carboxy methylcellulose sodium eye drops 1.0% and hypromellose 2.0% eye ointment ). Evaluation of serum plasma concentration of methotrexate was considered but it was not viable due to financial constraints.
The lesions started to heal after day 5 of hospitalisation. The dose of steroids was gradually tapered over two weeks; however, on day 16 patient developed right lower limb oedema. Venous Doppler of right lower limb revealed thrombosis of external iliac, common femoral, superficial femoral, popliteal and posterior tibial veins with diffuse subcutaneous oedema. A provisional diagnosis of deep vein thrombosis was made and subcutaneous injection of enoxaparin 40 mg was given for 7 days. A causality category of "possible" was assigned to this adverse event using the WHO-UMC scale. The case was registered with the Pharmacovigilance Programme of India.
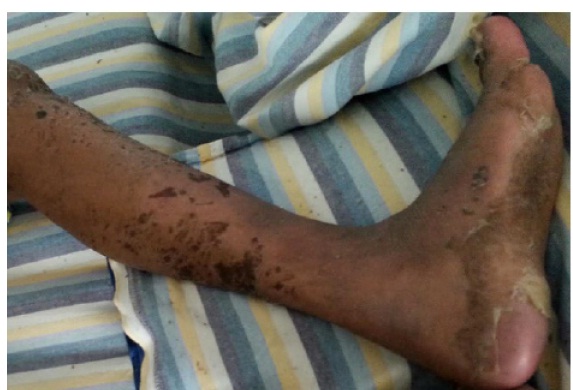
Sloughing-off of skin was noticed on palms and soles with the formation of new skin [Table/Fig-4].The patient was educated about the importance of always informing health care professionals about her allergic drug history when seeking treatment of rheumatoid arthritis in the future.
Large area of desquamation with erythema
Epidermal detachment tender underlying denuded skin over the neck, face and ear
The rash comprised of bullae in addition to erosions and peeling
Sloughing-off of skin noticed on soles with the formation of new skin at the follow up of one month
Discussion
The diagnosis and management of severe cutaneous adverse drug reactions like SJS and TEN require meticulous recording of the case history of the patient. The aetiology of SJS and TEN is not clear and could be due to drug induced immunological mechanism [1]. CD8 T-cells as well as the cytolytic molecules FasL and granulysin are key substances in the pathogenesis of SJS/TEN; however, the manner in which a culprit drug regulates the function of these key substances in a patient who develops SJS/TEN is the subject of ongoing research [1]. The most frequently involved groups of therapeutic agents cited in the literature are sulphonamides, anticonvulsants, non-steroidal anti inflammatory drugs and beta lactam antibiotics [2]. Although SJS and TEN are immune mediated hypersensitivity reactions, in our case it is debatable whether they are dose related or caused by drug-drug interaction.
Methotrexate has 100,000 times greater affinity than folic acid for the dihydrofolate reductase enzyme [3]. This mechanism of methotrexate reduce the tetrahydrofolate and attentuates the DNA synthesis in proliferating cells, making it an ideal disease modifying agent for rheumatoid arthritis. Methotrexate is metabolized into a series of polyglutamate derivatives that increase linearly with the concentration and duration of exposure. Therefore, higher doses of methotrexate administered for prolonged duration can result in greater toxicity [4]. MTX is reversibly bound to albumin in plasma and can be displaced by other drugs that are administered simultaneously [5]. Methotrexate is eliminated in its unchanged form (80%) via carrier mediated high organic anion transporter -3 (HOAT-3) in the basolateral membrane of proximal tubule [6]. Etoricoxib is a new NSAID that inhibits cyclooxygenase -2 (COX-2) with potent anti-inflammatory properties and good safety profile. Etoricoxib inhibits the HOAT-3 in a competitive and dose dependent manner [7] A recent Cochrane systematic review documented 17 publications on concurrent use of MTX and NSAIDs; however, none reported systemic adverse drug reactions or other major toxicity[8]. Though other non-steroidal anti-inflammatory drugs are implicated as one of the major causative agents for SJS and TEN, to the best of our knowledge there is only one case report of etoricoxib induced TEN that was reported by Kreft et al., [9].
Previously in few patients skin ulcerations, necrosis, erosions of the psoriatic plaques and bullous acral erythema, have been reported as adverse effects of methotrexate [10,11]. In a study, by Lawrence and Dahl, seven patients were treated with low dose of methotrexate and NSAIDs for psoriatic plaque and pre existing dermatitis. Ulcerations occurred in four patients, who received long term MTX therapy, either after increasing the MTX dosage or taking NSAIDs; the skin ulcerations healed after reducing MTX dose in these patients [11]. In the case discussed in our report, the patient received low dose methotrexate 7.5 mg weekly for a duration of six weeks before oral etoricoxib was added to her treatment regimen to alleviate pain. These medications were concomitantly taken for another six weeks. The patient presented with extensive skin necrolysis. This could be possibly due to high levels of methotrexate accumulation caused as a result of etoricoxib inhibiting HOAT-3 elimination of methotrexate at the renal tubule. Methotrexate being an anti-folate molecule may arrest normal epidermal cell proliferation at high doses. Thus, SJS and TEN may be a dose dependent adverse drug reaction in susceptible individuals. Inspite of MTX having a narrow therapeutic index, serum plasma concentration of MTX is not regularly monitored by the treating physicians. Folic acid supplementation is recommended along with methotrexate therapy by American College of Rheumatology to prevent the complications of methotrexate therapy [12,13]. Our patient had been taking folic acid 5mg once daily along with oral methotrexate.
Conclusion
Physicians should also consider the viability of diagnostic tests that directly assess the phenotype of the patient as these pharmacogenetic tests can utilise the genetic profiles of susceptible individuals to make treatment choices. This case highlights the importance of considering the risk of DDIs while treating the patient concomitantly with low dose methotrexate and newer generation COX-2 inhibitor etoricoxib.
[1]. T Harr, LE French, Toxic epidermal necrolysis and Stevens-Johnsons syndromeOrphanet J Rare Dis 2010 5(39):1-11. [Google Scholar]
[2]. B Jezierska-Krupa, L Hyla-Klekot, Toksyczna nekroliza naskörka a zespót [2]Stevensa-Johnsona (PolishPed Pol 1996 60:615-19. [Google Scholar]
[3]. GD Weinstein, G Goldfaden, P Frost, Methotrexate mechanism of action on DNA [3]synthesis in psoriasisArch Dermatol 1971 123:1303-07. [Google Scholar]
[4]. TA Madani, S Zafad, M Harif, A Quessar, S Benchekroun, Methotrexate-induced [4]toxic epidermal necrolysis: a case reportInternational Journal of Medicine and Medical Science 2009 1(4):99-101. [Google Scholar]
[5]. A Maeda, S Tsuruoka, K Ushijima, Y Kanai, H Endou, K Saito, Drug interaction [5]between celecoxib and methotrexate in organic anion transporter 3–transfected renal cells and in rats in vivoEur J Pharmacol 2010 640(1-3):168-71. [Google Scholar]
[6]. M Patane, M Ciriaco, S Chimirri, F Ursini, S Naty, RD Grembiale, Interactions among low dose of methotrexate and drugs used in the treatment of rheumatoid arthritisAdvances in Pharmaceutical Sciences 2013 http://dx.doi.org/10.1155/2013/313858 [Google Scholar]
[7]. H Honjo, Y Uwai, K Iwamoto, Inhibitory effect of selective cyclooxygenase-2 inhibitor etoricoxib on human organic anion transporter 3 (hOAT3Drug Metab Lett 2011 5(2):140-37. [Google Scholar]
[8]. AN Colebatch, JL Marks, DM van der Heijde, CJ Edwards, Safety of non-steroidal anti inflammatory drug and/or paracetamol in people receiving methotrexate for inflammatory arthritis: A Cochrane Systematic Review.J Rheumatol Suppl 2012 90:62-73. [Google Scholar]
[9]. B Kreft, J Wohlrab, I Bramsiepe, R Eismann, M Winkler, WC Marsch, Etoricoxibinduced toxic epidermal necrolysis: successful treatment with infliximabJ Dermatol 2010 37:904-06. [Google Scholar]
[10]. JR Roenigk HH, R Auerbach, HI Maibach, GD Weinstein, Methotrexate in psoriasis: revised guidelines.J Am Acad Dermatol 1988 19:145-56. [Google Scholar]
[11]. CM Lawrence, MG Dahl, Two patterns of skin ulceration induced by methotrexate in patients with psoriasis.J Am Acad Dermatol 1984 11(6):1059-65. [Google Scholar]
[12]. SL Morgan, JE Baggott, WH Vaughan, JS Austin, TA Veitch, JY Lee, Supplementation with folic acid during methotrexate therapy for rheumatoid arthritis: A double-blind, placebo controlled trialAnn Intern Med 1994 121(11):833-41. [Google Scholar]
[13]. SL Morgan, JE Baggott, WH Vaughn, PK Young, JV Austin, CL Krumdieck, The effect of folic acid supplementation on the toxicity of low-dose methotrexate in patients with rheumatoid arthritisArthritis Rheum 1990 33(1):9-18. [Google Scholar]