Pregnancy induced hypertension (PIH) includes a group of hypertensive disorders which develop during pregnancy and is one of the most common obstetric complication. PIH includes gestational hypertension, Pre-eclampsia and Eclampsia. Gestational hypertension is new onset hypertension developing after 20 weeks of pregnancy with blood pressure rising upto ≥ 140/90 mm Hg but without proteinuria. If it is not detected early, it can progress to Pre-eclampsia [1]. The cardinal features of pre-eclampsia are hypertension and proteinuria. If Pre-eclampsia progresses without proper treatment, patient eventually develops Eclampsia which is characterized by the presence of convulsions in association with the signs and symptoms of Pre-eclampsia. Hypertensive disorders complicate 5 to 10% of all pregnancies [1]. It has been estimated that worldwide about 76,000 pregnant women die each year from pre-eclampsia and related hypertensive disorders. As compared to women of a developed country, a woman in developing countries is seven times more likely to develop pre-eclampsia [2].
The aetiology of Pre-eclampsia is unknown but it is thought to be related to abnormal development of placenta. The normal endovascular invasion of cytotrophoblast into the spiral arteries fails to occur beyond decidua-myometrial junction. As a result, the musculoelastic media in the myometrial segment remains responsive to vasoconstrictor stimuli resulting in decreased blood flow. There is acute atherosis of spiral arteries with obliteration of lumen [3]. This results in under-perfusion of the placenta, localized ischemia and the onset of oxidative stress caused by excessive production of reactive oxygen species. These can in turn induce the formation of lipid peroxidation products. Both of these species are known to be the key mediators of systemic vascular dysfunction and inflammation [4]. Several studies have shown reduced maternal vascular endothelial function for months or even years after a pre-eclamptic pregnancy [5–7]. Pre-eclampsia and atherosclerosis have certain similarities; both are associated with dyslipidemia, endothelial dysfunction and an increase in the circulating levels of proinflammatory cytokines, such as interleukin-6 and tumour necrosis factor-α. An abnormal lipid profile is known to be strongly associated with atherosclerotic cardiovascular diseases and has a direct effect on endothelial dysfunction [8].
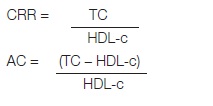
Several efforts have been made in seeking emergent or new cardiovascular risk factors to improve cardiovascular disease prediction. And, in an attempt to optimize the predictive capacity of the lipid profile, several lipoprotein ratios or “atherogenic indices” have been defined [9]. These indices could prove to be a better alternative to the routine investigations. One of them is CARDIAC RISK RATIO (CRR) which is frequently used for risk assessment of cardiovascular disease (CVD) and is given by the total cholesterol to HDL cholesterol ratio [10]. Another index is ATHEROGENIC INDEX OF PLASMA (AIP), calculated as log (TG/HDL-C). It has recently been proposed as a marker of plasma atherogenecity because it is increased in people at higher risk for coronary heart disease and is inversely correlated with LDL particle size. The association of TGs and HDL-C in this simple ratio theoretically reflects the balance between risk and protective lipoprotein forces, and both TGs and HDL-C are widely measured and available [11]. ATHEROGENIC COEFFICIENT (AC) is another index which is given by the ratio of non HDL cholesterol to HDL cholesterol. Non HDL-c is easily calculated, with no need for previous fasting of the patient. It is essentially the cholesterol analogue to an apo B level, having a higher correlation coefficient in comparison with the LDL cholesterol concentration [12]. In developed countries, atherosclerosis is the major cause of death and premature disability and is predicted to become the leading global cause of total disease burden by the year 2020 [13].
Research work on the topic and particularly on its long term complications is lacking in this region (Assam) where, lack of health awareness and education among women prevents them from seeking timely medical care during pregnancy. In view of this background, the present study has been undertaken in Gauhati Medical College and Hospital of Assam with the objectives to assess the fasting lipid profile in women diagnosed with pre-eclampsia as well as in women with normal pregnancy, to calculate their atherogenic indices and to correlate the findings of pre-eclamptic women with that of normal pregnant women in an attempt to utilize the data for the development of a new clinical approach for early recognition and prevention of risk of future cardiovascular diseases in women with pregnancy induced hypertension.
Materials and Methods
This was a case control study conducted in the Department of Biochemistry in collaboration with the Department of Obstetrics and Gynaecology at Gauhati Medical College & Hospital, Guwahati (Assam) between July, 2012 to July, 2013. The study included 50 primigravida women already diagnosed with pre-eclampsia in third trimester of pregnancy. They comprised the case group. Fifty age and gestational age matched healthy normotensive primigravida women with normal pregnancy were included in the control group. All the study subjects were in the age group 18-25 years and from diverse socio- economic status. A thorough history and physical examination was done and women with twin/multiple pregnancy, history of hypertension, diabetes mellitus, autoimmune disorders, renal, hepatic or thyroid gland diseases, intake of drugs which affect lipid metabolism, smoking and alcohol abuse were excluded from the study.
The study was approved by the Institutional Ethics Committee, vide letter no. MC/90/2012/pt-11/18. Informed consent was taken from all the women included in the study or their legal representative. The blood pressure was measured using a Mercury Sphygmomanometer and a stethoscope by palpatory and auscultatory methods. Taking all aseptic and antiseptic precautions 5ml of blood was drawn from the median cubital vein into the EDTA vacutainer tube. The blood was collected 12 to 14 hours after the last meal. The supernatant plasma from the vial was used for the investigations or transferred to clean dry vials for storage if the estimations were not done at the same sitting. However, all estimations were completed within 24 hours of collection of blood sample. Estimation of Lipid Profile was done using standard reagent kits after proper calibration of each method in MERCK microlab 300 Semiautoanalyser. Total Cholesterol (TC) measurement was based on the CHOD/PAP method,Triglyceride (TG) based on the GPO/PAP method, Direct LDL–cholesterol (dLDL-c) was estimated by kit based on the selective detergent method, HDL-cholesterol (HDL-c) by method based on precipitation by Phosphotungstate and VLDL by Friedewald’s formula.
Atherogenic indices were calculated by using the values of lipid profile parameters in the following way:
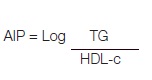
Where, concentration of TG and HDL are in mmol/L.
Calculation of AIP was done using CZECH online calculator of atherogenic risk [14].
Statistical Analysis
The results obtained were statistically analyzed using GraphPad InStat version 3.00. Statistical tests performed were student’s unpaired t-test and Mann-Whitney U-test (for Gestational age, because of the non normal distribution of its data). Correlations between the variables were estimated by Pearson’s correlation coefficients. Fisher’s-exact test was performed wherever applicable. The results were considered significant when the p-value was less than 0.05.
Results
Most of the subjects of control and case group were in the age group 20-24 years and gestational age 36-38 weeks. As can be seen from comparison of clinical characteristics, lipid profile parameters and atherogenic indices between the case (pre-eclampsia) and Control group depicted in [Table/Fig-1], there was a significant increase of systolic and diastolic blood pressure, AIP, CRR and AC in the case group as compared to the control group. Increase of lipid profile parameters was also significant in case group except for HDL which showed a non significant increase. There was a significant positive correlation of Atherogenic indices with blood pressure and gestational age in case group whereas, there was absence of any significant correlation in the control group. The results of pearson’s correlation are depicted in [Table/Fig-2,3,4]. Prevalence of pre-eclampsia was significantly higher in women with AIP>0.24, CRR>5, family history of pre-eclampsia and family history of hypertension than in women without such history. Fisher’s-exact test performed to find their odds ratio is represented in [Table/Fig-5].
Comparison of clinical characteristics, lipid profile parameters and atherogenic indices between the case (pre-eclampsia) and Control group
| Parameter | Mean | p-value |
|---|
| In Controls | In Cases |
|---|
| Age (years) | 23.84±3.530 | 24.1±3.688 | 0.7195 |
| Gestational age (weeks) | 35.96±1.784 | 36.48±1.418 | 0.1278 |
| Systolic Blood Pressure (mm Hg) | 117±5.002 | 161.96±8.136 | <0.0001 |
| Diastolic Blood Pressure (mm Hg) | 75.96±5.383 | 105.36±6.200 | <0.0001 |
| Total cholesterol(mg/dl) | 230.34±43.816 | 258.46±39.290 | 0.0010 |
| Triglycerides(mg/dl) | 222.62±49.133 | 254.2±43.671 | 0.0010 |
| Direct LDL (mg/dl) | 140.72±33.716 | 162.62±29.774 | 0.0008 |
| HDL(mg/dl) | 48.4±7.532 | 46.44±7.160 | 0.1854 |
| VLDL(mg/dl) | 44.5±9.871 | 50.84±8.702 | 0.0010 |
| AIP (Atherogenic index of plasma) | 0.2944±0.01178 | 0.3776±0.6592 | <0.0001 |
| CRR(Cardiac risk ratio) | 4.794±0.7805 | 5.61±0.09524 | <0.0001 |
| AC(Atherogenic coefficient) | 3.7908±0.7824 | 4.614±0.6782 | <0.0001 |
Representing correlation of different indices with systolic and diastolic blood pressure in pre-eclampsia (case) subjects
| Parameter | Systolic Blood Pressure | Diastolic Blood Pressure |
|---|
| r-value | p-value | r-value | p-value |
|---|
| AIP | 0.3583 | 0.0106 | 0.3144 | 0.0261 |
| CRR | 0.3137 | 0.0265 | 0.5035 | 0.0002 |
| AC | 0.3193 | 0.0238 | 0.5068 | 0.0002 |
[r- Pearson’s coefficient]
Representing correlation of different indices with systolic and diastolic blood pressure in normal pregnancy(control) subjects
| Parameter | Systolic Blood Pressure | Diastolic Blood Pressure |
|---|
| r-value | p-value | r-value | p-value |
|---|
| AIP | 0.1262 | 0.3825 | 0.05282 | 0.7156 |
| CRR | 0.1231 | 0.3945 | 0.08327 | 0.5653 |
| AC | 0.1151 | 0.4261 | 0.0750 | 0.6047 |
Representing correlation of the three indices with gestational age in pre-eclampsia (case) subjects
| Parameter | r-value | p-value |
|---|
| AIP | 0.2877 | 0.0427 |
| CRR | 0.3283 | 0.0199 |
| AC | 0.3311 | 0.0189 |
Representing results of Fisher’s-exact test, performed to find odds ratio
| Risk Factors | Pre-eclampsia (%) | Healthy Pregnancy (%) | p-value | Odds ratio | 95% CI |
|---|
| AIP>0.24 | 48 | 36 | 0.0019 | 9.333 | 1.993 to 43.698 |
| AIP≤0.24 | 2 | 14 |
| CRR>5 | 39 | 19 | <0.0001 | 5.785 | 2.400 to 13.945 |
| CRR≤5 | 11 | 31 |
| Family H/O of hypertension | 19 | 9 | 0.0440 | 2.792 | 1.112 to 7.009 |
| Family H/O of preclampsia | 8 | 2 | 0.0208 | 6.00 | 1.196 to 30.112 |
Discussion
During pregnancy, maternal metabolic environment is modified by the alterations in maternal hormones and a pronounced hyperlipidemia is considered as physiological. But in pre-eclampsia, these changes in lipid metabolism are exaggerated and are a major cause of endothelial dysfunction that is reduced vasodilation and anticoagulant properties as well as increased adhesion molecule expression, cytokine release and reactive oxygen species production from the endothelium. In the present study, the mean gestational age of patients was 36.48 weeks with a standard deviation of ±1.418 weeks and is similar with the finding of Sahu et al., (36.3±2.9 weeks) and Cekman et al., (36.4±1.2 weeks) [15,16]. There was a significant difference in systolic as well as diastolic blood pressure between cases and controls (p<0.0001). These findings are comparable to the findings of Cekmen et al., (mean diastolic blood pressure was 79±1.9 in the control group and 106±2.2 in the case group and systolic blood pressure was 119±3.1 in the control group and 157±3.6 in the case group) [16].
In the current study we calculated the atherogenic index of plasma (AIP). The mean of AIP in case group was higher than that of control group and was found to be extremely significant (p<0.0001). AIP calculated as log (TG / HDLc) has been proposed as a marker of plasma atherogenicity [17]. From our findings we can say that plasma lipids in normal pregnancy are at atherogenic levels, and more so in pre-eclampsia.
It has been suggested that AIP values above 0.24 are associated with high cardiovascular risk [18]. We performed Fisher-exact test for this. The odds ratio for women with AIP >0.24 was 9.333 (95% confidence interval=1.993-43.698, p<0.0019) when compared with women with AIP < 0.24. This means women with AIP>0.24 have pre-eclampsia 9.333 times as often as women with AIP<0.24.
The Cardiac risk ratio (TC/HDL) has been found to be significantly elevated in preeclamptic women in studies conducted in India [8,19] as well as outside [20]. In the current study, we also noted a significantly (p=<0.0001) increased TC:HDL ratio in the case group. On performing the Fisher-exact test, we found that women with CRR > 5 have pre-eclampsia 5.785 times as often as women with CRR < 5 (O.R=5.785, 95% confidence interval=2.400-13.945, p<0.0001). NCEP ATP III [21] use the TC/HDL ratio as a powerful predictor of CV risk. The mean of atherogenic coefficient (AC) in the case group (4.614±0.6782) was higher than that of control group (3.7908±0.7824) and was found to be extremely significant (p<0.0001). Non-HDL cholesterol is said to be a suitable surrogate marker for total apolipoprotein B because of its high correlation with the apolipoprotein B levels. However, in routine clinical practices standardized measurements of apolipoprotein B are not always available [21]. So, this simple ratio of non HDL (atherogenic) and HDL (atheroprotective) cholesterol could prove valuable in identifying any increased cardiovascular risk.
Our results of present study allow us to hypothesize that the dyslipidemia in pre-eclampsia and the associated lesions may evoke adverse cardiovascular events in latter life of these women. Infact, Bellamy et al., in a systematic review and meta-analysis reported that women with a past history of pre-eclampsia present increased risk of hypertension (relative risk, RR= 3.7) [22], venous thromboembolism (RR = 1.79) and stroke (RR = 1.81). These findings confirm the possible association between hypertension during pregnancy and future cardiovascular disease. Lipids can cause hypertension by means of oxidative stress. Oxidative stress promotes vascular smooth muscle cell proliferation and hypertrophy and collagen deposition, leading to thickening of the vascular media and narrowing of the vascular lumen [23]. Also, Lipid peroxides causes stimulation of thromboxane synthesis and inhibition of endothelial derived relaxing factor (EDRF) [24] which leads to vasoconstriction. Usha et al., and Bhardwaj et al., in their respective studies on women with pre-eclampsia have concluded that dyslipidemia leads to oxidative stress in pre-eclampsia and could lead to increased atherogenecity in these women [25,26].
Pearson’s correlation was derived between atherogenic indices and blood pressure in both the groups and it was found that the indices had a statistically significant positive correlation with systolic as well as diastolic blood pressure in case group, whereas in control group the association was not significant. There is a dearth of research work on the study of these atherogenic indices in pre-eclampsia patients. Some authors have studied correlation of lipid parameters with blood pressure in pre-eclampsia patients. Kashinakunti et al., and Bennal et al., found a positive correlation between seum triglycerides and systolic blood pressure, and also between diastolic blood pressure and serum triglycerides [27,28]. Adewolu in his study conducted in Benin city [29], Nigeria noticed a positive correlation of Triglycerides, total cholesterol, LDL cholesterol with diastolic blood pressure. In the same study he found that total cholesterol values correlate significantly (r=.555, p<0.05) with gestational age also, with increasing gestational age, total cholesterol values increased. In the current study we looked for a correlation between atherogenic indices and gestational age in the case group and found each of the three indices to be positively and significantly correlated with gestational age (AIP: r=0.2877,P=0.0427 ; CRR: r=0.3283,p=0.0199 ; AC: r=0.3311, p=0.0189). In our study, 19% of the preeclamptic women gave a family history of hypertension and were noted to suffer from pre-eclampsia 2.7 times as often as women who didn’t have such history (O.D=2.792, C.I=1.112-7.009, p=0.0440). This finding is supported by Magnussen et al., who found in their population based cohort study that family history of hypertension is associated with doubled risk of PIH [30].
In a systematic review of controlled studies published between 1966 to 2002, Duckitt and Harrington found that a family history of pre-eclampsia nearly triples the risk of pre-eclampsia [31]. In our study 8% of the cases gave a family history of Pre-eclampsia. And after doing the Fisher-exact test, we found that such women suffer from pre-eclampsia six times as often as women without such history (O.R= 6.00, 95% C.I=1.196-30.112, p= 0.0208). The reason for overexpression of this risk in our study could be the smaller size of our study group.
The pattern of lipid abnormalities noticed in pre-eclampsia may predispose these women to future cardiovascular diseases. Barden et al., assessed lipid and lipid peroxidation six weeks postpartum in preeclamptic women and found that dyslipidemia and lipid peroxidation was persistent even after delivery [32]. Moreover, several authors have also found decreased endothelial function in the blood vessels of women even many years after a preeclamptic pregnancy [5,6]. Another important fact is that in previous studies, vascular lesions in the placental bed of women with pre-eclampsia were reported to be similar to atherosclerotic plaques [33].
It has been found that premature CVD-related deaths are higher in Asian Indians [34]. The first myocardial infarction (MI) attack occurs in 4.4% of Asian women at age less than 40 years, which is 2- to 3.5- fold higher than in the West European population [35]. Therefore, it can be said that such ratios, better called atherogenic indices may be more appropriate to be used to assess the relative contribution of lipids to the cardiovascular risk in pre-eclampsia patients than individual lipid parameter measurements and could help in identifying a subset of population who might benefit from heightened surveillance and early preventive intervention.
Limitations
Our study has some limitations that are the inability to follow up the subjects with pre-eclampsia, due to paucity of time and limited resources. Also there was no lipid profile evaluation done in pre pregnancy state of the subjects.
Conclusion
The present study substantiates the growing numbers of studies showing that dyslipidemia in pre-eclamptic women turn out to be an increased risk factor for cardiovascular complications. The significance of increased values of atherogenic indices in pre-eclamptic pregnancy as compared to normal pregnancy cannot be ignored as they point towards increased threat in women with pre-eclampsia as they imply an increased threat of developing CVD in women with pre-eclampsia. The present study will encourage new studies related to the above topic, conducted on a larger study group and covering the aspects of a pre-pregnant lipid profile evaluation and follow-up.
[r- Pearson’s coefficient]