Introduction
Oral mucosa is continuously exposed to a wide variety of injurious agents such as tobacco, tobacco smoke, alcohol, areca nut, and betel quid or pan etc [1] . Many kinds of diseases appear on the oral mucosa due to various factors, and some display clear dispositions toward certain sites. Cell mediated immunity is thought to bear some relationship to these oral diseases. For cell mediated immunity, T cell must be sensitized by information from antigen presenting cell (APC), such as langerhans cells (LCs) [2] . LCs are immunocompetent cells residing within the oral mucosa which, together with intraepithelial lymphocytes, play a role in mucosal defence [3] .
LCs originates from the bone marrow and then migrates into the epithelium to perform the function of antigen recognition and presentation. Myeloid progenitors give rise to precursors of LCs [4] . LCs were thought to be closely involved in Oral Lichen Planus (OLP) and Oral Squamous Cell Carcinoma (OSCC) which can be detected by immunohistochemical analysis using S100 protein as a marker. These proteins are called S100 because of their solubility in a 100%-saturated solution with ammonium sulphate at neutral pH [5] .
The present study was designed to assess and compare langerhans cells immunohistochemically in normal mucosa, OLP and OSCC using anti S100 antibody and to know whether LCs play any role in local immune response to these diseases.
Materials and Methods
The present study comprised of 30 cases of previously diagnosed OLP, 35 cases of OSCC (15 well differentiated, 14 moderately differentiated, 6 poorly differentiated), that were randomly selected from the archives (2011-2013,and the data was assessed in 2013)of Department of Oral Pathology, Kamineni Institute of Dental mavath6Sciences, along with control group consisting of 30 normal healthy mucosa, who came for routine dental check up. A verbal consent was taken. Paraffin wax embedded tissue blocks of 30 (OLP) and 35 (OSCC) were sectioned to 3μm thickness with rotary microtome. The normal healthy oral mucosa of 30 individuals was obtained, fixed, processed, embedded with wax and were also sectioned to the same thickness of 3μm. All the sections (both study groups and control groups) were taken onto superfrost plus glass slides, deparaffinised by placing the slides in the hot air oven at 100ºC for 10 minutes and further rehydrated by taking the tissue sections through two changes of xylene, absolute alcohol, 80% alcohol, and 70% for 5 minutes each. The slides were immersed under running tap water for 2 to 5 minutes.
Antigen Retreival
The slides were placed in a coupling jar with Tris buffered saline solution which in turn was kept in a microwave oven and heated for four to five times at 100ºC temperature for a period of 5 minutes each. All the slides were allowed to cool to room temperature and all the reagents stored in the refrigerator were brought to room temperature (24º-28ºC) prior to immunohistochemical staining. At no time the tissue sections were allowed to dry during the staining procedure. The sections were then washed gently with phosphate buffer solution (PBS) three to four times for two minutes each and excess of buffer solution was tapped off.
The sections were then covered with peroxide block for 15-20 minutes and washed gently with PBS three to four times for two minutes each. After tapping off the excess buffer from the slide, the sections were covered with Power Block for 15-20 minutes. Then the sections were covered with pre-diluted S100 primary antibody (BioGenex) and incubated for one hour at room temperature and then washed gently with PBS three to four times for two minutes each.
Super Enhancer (a reagent which enhances the reaction) was added to the tissue sections and left for 30 minutes followed by gentle washing with PBS three to four times for two minutes each. After tapping off the excess buffer, the tissue sections were then incubated with secondary antibody for 30 minutes and washed gently with PBS three to four times for 2 minutes each. Excess buffer was tapped off and tissue sections were covered with freshly prepared substrate Diaminobenzidine (DAB) chromogen solution for 10 minutes followed by gentle washing with distilled water for two minutes. The sections were then immersed in Harri’s haematoxylin for two minutes and washed gently under running tap water for bluing. Finally the tissue sections were dehydrated through series of absolute alcohol, 80% alcohol, 70% alcohol for 5 minutes each respectively. Then the sections were immersed in xylene for clearing and later mounted by using DPX.
Results
After the tissue sections were stained immunohistochemically by using anti S100 antibody in each group for detection of LCs. Histomorphometric quantification of LCs was performed by counting the cells that were S100-positive. The LCs were based on nucleic and cytoplasmic staining and their dendritic shape. S100 positive cells in basal layers were excluded as probable melanocytes. The S100 positive LCs were counted in 3 to 5 fields, that were selected randomly, under 100X magnification. Presence of brown coloured end product at the site of target antigen was indicative of positive immunoreactivity. The antibody stained positive for the cytoplasm and nucleus of cells in the tissue sections.
SPSS (Statistical package for social sciences) soft ware 19 version was used for statistical analysis. The significance of the results obtained from the control and study groups were statistically analysed by one-way Anova test. Multiple comparisions between the groups were assessed for statistical significance using Tukey Kramer honestly significant difference (HSD) test. The p-value <0.05 was considered to be statistically significant. Mean and standard deviation of cases and controls were also determined.
The mean value of number of LCs between the study and control groups were tabulated. The mean value of number of LCs in controls was 6.5, in OLP the mean value was 25.7333 and in OSCC was 24.9722 [Table/Fig-1].The data were assessed for significance using one way ANOVA and p-value was 0.001 which was statistically significant [Table/Fig-2]. Multi parametric TUKEY HSD test was done between controls, OLP, and OSCC. The results indicate the p-value was found to be statistically significant between controls and the categorized groups (OLP and OSCC), but not significant between OLP and OSCC [Table/Fig-3,4,5].
a. Controls Vs categorized groups, p-values:
Controls Vs oral lichen planus – .000 (statistically significant)
Controls Vs oral squamous cell carcinoma – .000 (statistically significant)
b. Within the categorized groups, p-values:
Oral lichen planus Vs OSCC–0.987 (statistically not significant)
The mean value of number of langerhans cells in well differentiated SCC was 33.4667, in moderately differentiated SCC was 23.2143, and in poorly differentiated SCC was 10.3333 [Table/Fig-6].The data were assessed for significance using one-way ANOVA and p- value was 0.001, which was statistically significant [Table/Fig-7]. Multi parametric TURKEY HSD test was done between three groups of squamous cell carcinoma (well, moderately, and poorly differentiated). The results indicate that p-value was found statistically significant between the groups [Table/Fig-8,9,10,11].
a. Within the categorized groups, p-value:
WSCC Vs MSCC - 0.001 (statistically significant)
WSCC Vs PSCC -0.001 (statistically significant)
MSCC Vs PSCC -0.001 (statistically significant)
Discussion
LCs originates from the bone marrow and then migrates into the epithelium to perform the function of antigen recognition and presentation. Myeloid progenitors give rise to precursors of LCs [5] which play a role in the pathogenesis of lichen planus, a chronic mucocutaneous disorder thought to result from cell-mediated immune damage. Light and electron microscope observations suggest the earliest detectable change in lichen planus is an intraepithelial accumulation of LCs [2] . Langerhans cells were believed to play an important role in tumour immunology by emergence of new antigens which are involved in malignant transformation [6] . During the initiation, promotion, and progression of carcinogenesis, changes occur in the specific host immunological factors [7] .The major cells which play an important role in killing cancer are the T lymphocytes. However, these T cells need to be activated upon antigen presentation, which is mediated by the antigen presenting cells, one of which is the langerhans cell [6] . A suggested possibility in the cancer is that these langerhans cells will be presenting an tumour antigens to the lymphocytes [7] .
In the present study the results showed that there was significant increase in the mean value of number of langerhans cells (in the study groups i.e. OLP and OSCC when compared to that of control group (normal healthy mucosa) which was statistically significant (p=0.001).The results of our study also revealed that there was no statistically significant change in mean value of number of langerhans cells between the study groups i.e. oral lichen planus and oral squamous cell carcinoma (p=0.987).
The mean value of number of LCs in control group was 6.5, and in oral lichen planus was 25.7333, which was statistically significant (p=0.001).This may be due to changes in the regulation of local immune reaction, which might result in an increase in number of LCs in OLP, which correlates with the study of Toto et al., Amerigo Santoro et al., Sloberg et al., and Gueiros LA et al., [8-11] .
The mean value of number of LCs in controls was 6.5, and in OSCC was 24.9722, which was statistically significant (p=0.001). This increase in mean value of number of LCs in OSCC group when compared to control group may be attributed to the following reasons: LCs are antigen presenting cells to lymphocytes in skin or oral mucosa. As already mentioned above, a suggested possibility in cancer is that, the LCs present tumour antigens to the lymphocytes [12,13] , which also correlates with the study of Kurihara et al., [13] and De La mater [7] .
There was also significant change in mean value of number of LCs among OSCC groups i.e. well differentiated, moderately and poorly differentiated which was statistically significant (p=0.001). The mean value of number of langerhans cells in well differentiated squamous cell carcinoma was 33.4667, in moderately differentiated squamous cell carcinoma was 23.2143, and in poorly differentiated squamous cell carcinoma was 10.3333.This decrease in mean value of langerhans cells compared within OSCC groups could 78be due to immune suppression which was induced by anaplastic tumour cells and LCs tending to undergo apoptosis promotes a substantial decrease in the amount of LCs population [14] , which correlates with the study of Upadhyay J et al., [15] .
The difference in mean value of LCs in OLP and OSCC may be due to threshold densities of LCs. The LCs are required for antigen specific T-cell activation in oral lichen planus leading to higher number of LCs in OLP whereas immunological impairment in invasive squamous cell carcinoma leads to a lower number of LCS. However it is interesting to note that tumour promoters, but not initiators, deplete langerhans cells and further loss of LCs during tumour promotion impairs the normal immunological functions. This could be the reason for the decrease in mean value of langerhans cells from well differentiated squamous cell carcinoma to poorly differentiated squamous cell carcinoma [16] .
The results of our study indicated that the mean value of LCs increased in both OLP and OSCC when compared to the control group. As mentioned earlier changes in integrity of the immune system may result in changes in the LCs which could be the reason for increase in number of langerhans cells in OLP and OSCC.
Descriptive analysis between the groups
| Groups | N | Mean | Std. Error of Mean | Median | Mode | Std. Deviation | Range | Minimum | Maximum |
|---|
Control
OLP OSCC
| 30
30 35
| 6.5000
25.7333 24.9722
| .42548
1.84324 1.77526
| 6.0000
24.0000 26.0000
| 5.00
21.00 26.00
| 2.33046
10.09586 10.65159
| 8.00
39.00 37.00
| 3.00
7.00 5.00
| 10.00
46.00 42.00
|
OLP- oral lichen planus, OSCC- oral squamous cell carcinoma
One-way ANOVA table between the groups
| Sum of Squares | df | Mean Square | F | Sig. |
|---|
| Between Groups | 7453.854 | 2 | 3726.927 | 50.028 | 0.001 |
| Within Groups | 6853.767 | 92 | 74.497 | | |
| Total | 14307.621 | 94 | | | |
Multiple Comparisons Turkey HSD table between all the three groups
| (I) gp | (J) gp | Mean Difference (I-J) | Std. Error | Sig. | 95% Confidence Interval |
|---|
| Lower Bound | Upper Bound |
|---|
| 1.00 | 2.00 | -19.23333* | 2.22856 | 0.001 | -24.5423 | -13.9244 |
| 3.00 | -18.90000* | 2.14750 | 0.001 | -24.0158 | -13.7842 |
| 2.00 | 1.00 | 19.23333* | 2.22856 | 0.001 | 13.9244 | 24.5423 |
| 3.00 | .33333 | 2.14750 | 0.987 | -4.7825 | 5.4492 |
| 3.00 | 1.00 | 18.90000* | 2.14750 | 0.001 | 13.7842 | 24.0158 |
| 2.00 | -.33333 | 2.14750 | 0.987 | -5.4492 | 4.7825 |
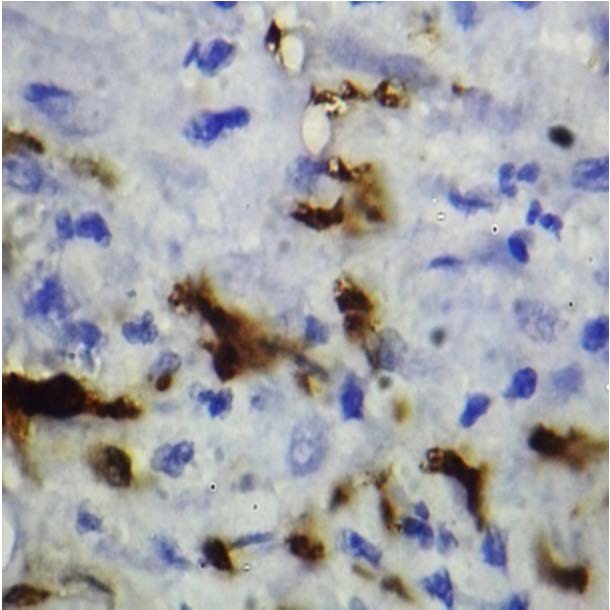
Langerhans Cells In Normal Mucosa-100x.
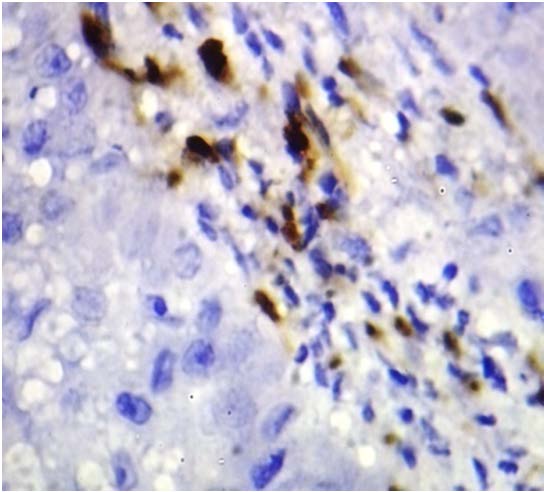
Langerhans Cells In Oral Lichen Planus-100x
Descriptive analysis between squamous cell carcinoma groups
| Groups | N | Mean | Std. Error of Mean | Std. Deviation | Variance | Range | Minimum | Maximum |
|---|
WSCC
MSCC PSCC
| 15
14 6
| 33.4667
23.2143 10.3333
| 1.55798
1.95886 2.06020
| 6.03403
7.32938 5.04645
| 36.410
53.720 25.467
| 19.00
24.00 14.00
| 23.00
12.00 5.00
| 42.00
36.00 19.00
|
WSCC-well differentiated squamous cell carcinoma, MSCC-moderately differentiated
One-way ANOVA table between oral squamous cell carcinoma groups
| Sum of Squares | df | Mean Square | F | Sig. |
|---|
| Between Groups | 2635.453 | 2 | 1317.727 | 32.560 | .000 |
| Within Groups | 1335.519 | 33 | 40.470 | | |
| Total | 3970.972 | 35 | | | |
Multiple Comparisons Turkey HSD between squamous cell carcinoma groups
| (I) GP | (J) GP | Mean Difference (I-J) | Std. Error | Sig. | 95% Confidence Interval |
|---|
| Lower Bound | Upper Bound |
|---|
| WSCC | MSCC | 10.25238* | 2.36405 | 0.001 | 4.4515 | 16.0533 |
| PSCC | 23.18095* | 2.91196 | 0.001 | 16.0356 | 30.3263 |
| MSCC | WSCC | -10.25238* | 2.36405 | 0.001 | -16.0533 | -4.4515 |
| PSCC | 12.92857* | 2.94486 | 0.001 | 5.7025 | 20.1547 |
| PSCC | WSCC | -23.18095* | 2.91196 | 0.001 | -30.3263 | -16.0356 |
| MSCC | -12.92857* | 2.94486 | 0.001 | -20.1547 | -5.7025 |
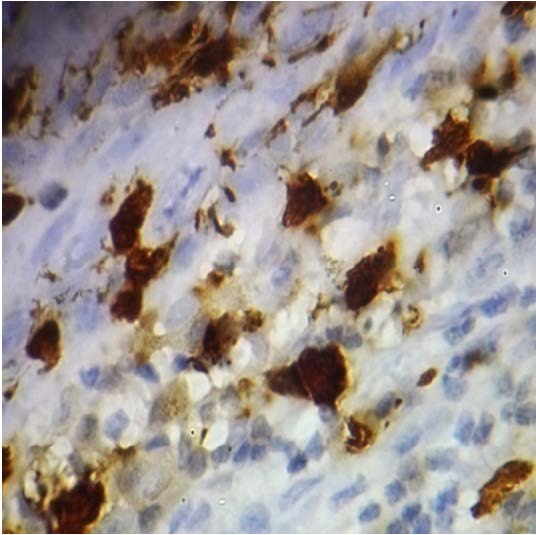
Langerhans Cells in Well Differentiated Squamous Cell Carcinoma -100x
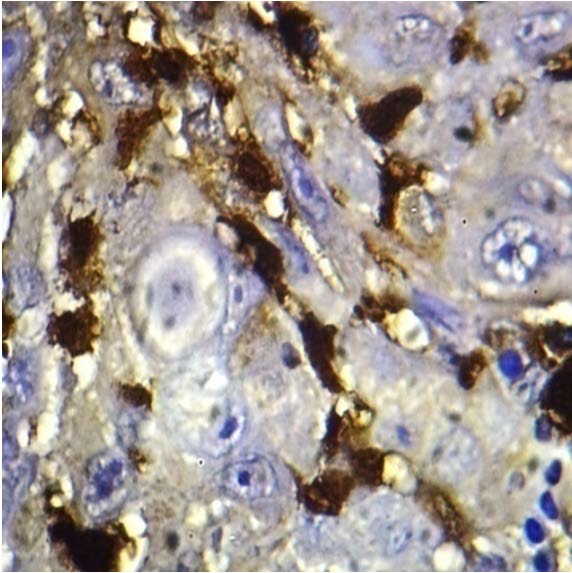
Langerhans Cells in Moderately Differentiated Squamous Cell Carcinoma -100x.
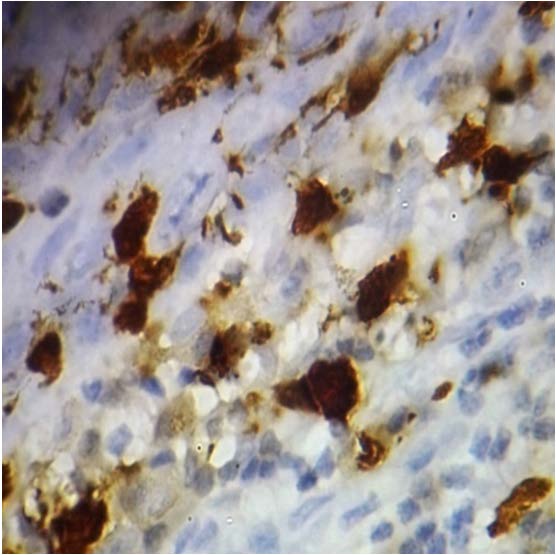
Langerhans Cells in Poorly Differentiated Squamous Cell Carcinoma -100x
Conclusion
LCs, as antigen presenting cells play a significant role in various oral pathologic conditions including OLP and OSCC. Changes in the integrity of the immune system during the course of OLP and OSCC may result in change in the LCs. A better understanding and clarity of LCs is pivotal for designing novel or improved therapeutic approaches that will allow proper functioning of LC’s in patients with OLP and OSCC, thus significantly reducing the morbidity of OLP and OSCC patients. Further investigation is required with larger sample size and with other conditions that would predict the immunological significance of LCs.
OLP- oral lichen planus, OSCC- oral squamous cell carcinoma
WSCC-well differentiated squamous cell carcinoma, MSCC-moderately differentiated
[1]. A Narwal, SA Saxena, Comparision of langerhans cell numbers in tobaccoassociated leukoplakia and oral squamous cell carcinomaJ Oral Med Sci 2011 10(3):125-31. [Google Scholar]
[2]. K Mogi, T Yamaguchi, Histopathological study on the distribution of langerhans cells in oral mucosaKitakanto Med J 2007 57(4):307-10. [Google Scholar]
[3]. PM Farthing, P Matear, AT Cruchley, The activation of Langerhans cells in oral lichen planusJ oral pathol med 1990 19(2):81-85. [Google Scholar]
[4]. CW Cutler, R Jotwani, Dendritic Cells at the Oral Mucosal InterfaceJ Dent Res 2006 85(8):678-89. [Google Scholar]
[5]. I Marenholz, CW Heizmann, G Fritz, S100 proteins in mouse and man: from evolution to function and pathology (including an update of the nomenclature)Biochem Biophys Res Commun 2004 322(4):1111-22. [Google Scholar]
[6]. KM Pereira, RC Soares, MC Oliveria, LP Pinto, L Costa Ade, Immuno histochemical staining of langerhans cells in HPV positive & HPV negative cases of oral squamous cell carcinomaJ oral sci 2011 19(4):378-83. [Google Scholar]
[7]. RB Upadyay, J Upadyay, NN Rao, P Agarwal, Role of langerhans cells in oral squamous cell carcinomaThe Chinese-German Journal of Clinical Oncology 2011 10(10):606-11. [Google Scholar]
[8]. PD Toto, HT Nadimi, An Immunohistochemical Study of Oral Lichen PlanusOral Surg Oral Med Oral Pathol 1987 63(1):60-67. [Google Scholar]
[9]. A Santoro, A Majorana, L Roversi, F Gentili, S Marelli, Recruitment of dendritic cells in oral lichen planusJ of Pathol 2005 205(4):426-34. [Google Scholar]
[10]. K Sloberg, R Jonsson, M Jontell, Assessment of Langerhans cells in oral lichen planus using monoclonal antibodiesJournal of Oral Pathology & Medicine 1984 13(5):516-24. [Google Scholar]
[11]. LA Gueiros, R Gondak, J Jorge Junior , RD Coletta, Increased number of Langerhans cells in oral lichen planus and oral lichenoid lesionsOral Surg Oral Med Oral Pathol Oral Radiol 2012 113(5):661-66. [Google Scholar]
[12]. S Jaitley, TR Saraswathi, Pathophysiology of langerhans cellsJournal of oral and maxillofacial pathology 2012 16(2):239-44. [Google Scholar]
[13]. N Mizukawa, K Sugiyama, E Yamachika, T Ueno, K Mishima, T Sugahara, Presence of defensin in epithelial Langerhans cells adjacent to oral carcinomas and precancerous lesions Anticancer Res 1999 19(4B):2969-71. [Google Scholar]
[14]. LC Ricardo, RL Albuquerque, MC Miguel, AL Costa, LB Souza, Correlation of c-erbB-2 and S-100 expression with the malignancy grading and anatomical site in oral squamous cell carcinomaInt J Exp Pathol 2003 84(4):259-65. [Google Scholar]
[15]. J Upadhyay, NN Rao, RB Upadhyay, A comparative analysis of langerhans cell in oral epithelial dysplasia and oral squamous cell carcinoma using antibody CD- 1aJ Cancer Res Ther 2012 8(4):591-97. [Google Scholar]
[16]. P Arachchi, J Cranei, C Scully, SS Prime, Epithelial Dendritic Cells in Pathological Human Oral TissuesJ oral pathol med. 1989 18(1):11-16. [Google Scholar]