Introduction
Oral Squamous Cell Carcinoma (OSCC) is the sixth most common cancer in the world and is one of the leading causes of death in India. It is a malignant neoplasm arising from the oral mucosal epithelium. It constitutes upto 90% of all oral malignancies and remains a serious oral health problem worldwide [1]. OSCC has a complex biological behaviour and despite advancements in diagnostic aids and therapeutic improvements, prognosis of patients with OSCC remains poor and the five year survival rate is still below 50% [2]. OSCC occurs because of discrete molecular changes occurring due to loss of genomic integrity following continuous exposure to environmental risk factors which includes predominantly tobacco consumption [3]. Tumour aggressiveness is variable in patients and is controlled by various biological markers. Importance of biological markers (oncogenes, cell cycle related molecules, growth factors, angiogenesis related factors) in predicting prognosis and response to the treatment is increasing. Many molecular biological markers have been extensively studied to aid in the prevention and prognosis of OSCC. However, no marker has been universally accepted so far [4].
Angiogenesis or neovascularisation is the formation of new blood vessels from pre-existing ones. It is an important component in many biological processes, both in physiological conditions, such as proliferating endometrium and embryogenesis and in pathological conditions such as rheumatoid arthritis and neoplastic diseases [5]. It is a complex activity that is required for continuous growth and survival of tumours. The growth of tumours beyond a few millimeters depends on the formation of new blood vessels. Angiogenesis has long been known to aid progression and metastasis of many malignant tumours including those of the oral cavity [6]. Angiogenesis is a complex process regulated by a balance between angiogenic stimulators like fibroblast growth factor (FGF), transforming growth factor-beta (TGF-β), vascular endothelial growth factor (VEGF), and inhibitors like angiostatin, platelet factor IV (PF4), and thrombospondin-1 (TSP-1) [7]. Angiogenesis can be triggered by hypoxia, caused by increasing distance of growing tumour cells from the blood vessels [8]. Also, several oncogenes like V-ras, K-ras, V-raf, etc. have the tendency to up-regulate angiogenic factors like VEGF and increase the production of cytokines and proteolytic enzymes [9].
Angiogenesis is mediated by angiogenic factors released by both the oncogenic cells as well as the host immune cells. Among the various host immune cells, mast cells have been implicated in tumour progression by promoting angiogenesis [10]. Mast cells are phylogenetically old, highly granulated, already known for their key role in type I hypersensitive reaction. These are highly engineered cells which can be activated by number of various stimuli. Following activation by immunological or non-immunological stimuli, mast cells release via their granules a range of tissue mediators like histamine, heparin, tryptase, chymase, basic fibroblast growth factor, etc. which have the tendency to induce angiogenesis [11]. Though mast cells have been established in promoting angiogenesis in various malignant tumours, little is known of its role in OSCC [12].
Some studies have been done to correlate mast cells and angiogenesis in OSCC, but all these studies show controversial results [13-16]. Therefore, in this study we analysed blood vessel density (BVD) using CD34 marker and counterstaining the same section on the slide with a mast cell specific stain i.e. Toluidine blue stain for analysing mast cell density (MCD), to get better results.
Materials and Methods
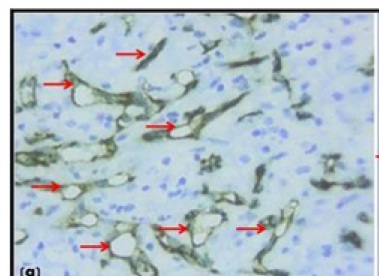
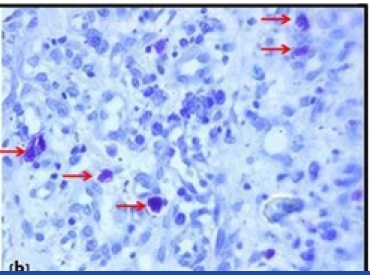
Material for the study included 38 formalin fixed paraffin embedded tissue blocks of different grades of OSCC and normal oral mucosa, which was used as control (11 well-differentiated, 13 moderately differentiated, 4 poorly differentiated & 10 normal oral mucosa). Also, paraffin embedded tissue blocks of pyogenic granuloma were used for the staining standardization [Table/Fig-1a,1b]. The blocks were retrieved from the archives of the Department of Oral Pathology and Microbiology, Sinhgad Dental College and Hospital, Pune, India. The study was approved by Institutional Review Board (IRB). Immunohistochemical analysis for blood vessels was performed using CD34 marker (Novolink- Min Polymer Detection system, Leica).
1) 3μm sections were cut and taken on a APES coated slide
2) Slides were kept for overnight incubation at 37˚C
3) Section were dewaxed in Xylene
4) Sections were brought to distilled water through graded alcohols
5) Pressure cooker technique was used for antigen-retrieval
6) Slides were washed with tris-buffer solution (pH- 7.6 ) during the procedure
7) Novolink- Min Polymer detection system, Leica was used
8) The sections were then counter stained with mast cell specific stain: 1% Toluidine Blue stain
9) Sections were mounted with DPX and cover slipped.
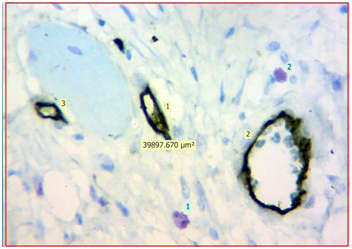
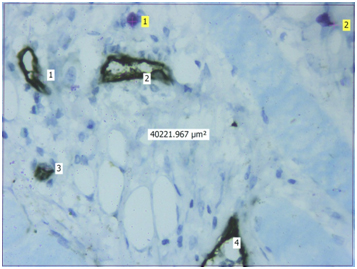
Evaluation: Evaluation of IHC and toluidine blue staining was performed both qualitatively and quantitatively using Leica Research microscope. Initial evaluation was qualitative in order to examine different expression pattern of the CD34 antibody. For quantitative analysis, stained sections were seen using 40X magnification to identify the areas of highest vascularisation and mast cells. Four such fields fulfilling the above criteria were chosen. Blood vessels and mast cells counts were performed in these 4 chosen fields using Leica application suite software (LAS v4.5). The blood vessels were identified by the presence of brown stained endothelial cells around them. Similarly, mast cells were identified as purplish granulated areas in a light blue background. Blood vessel density (BVD) and Mast cell density (MCD) was calculated from each 4 fields using formula mentioned below. Mean BVD and MCD was then given for the section analysed.
Blood vessel density (BVD): Blood vessel density was calculated from each 4 fields using the formula-
BVD= No. of blood vessels in a field/ Area of a field = No. of blood vessels in a field/ 0.04mm2 (Area of field i.e. 40X magnification= 0.04 mm2)
Mast cell density (MCD ): Mast cell density was calculated from the same 4 fields using the formula-
MCD= No. of mast cells in a field/ Area of a field = No. of mast cells in a field/ 0.04mm2 (Area of field i.e. 40X magnification= 0.04 mm2)
Results
Blood vessel density (BVD): All the 38 cases including OSCC and normal oral mucosa tested for vascularisation were immunopositive for CD34. Immunohistochemical staining of CD34 was evaluated within the endothelial cells which stained positively. When the blood vessel density (BVD) was evaluated in normal oral mucosa [Table/Fig-2], well-differentiated OSCC [Table/Fig-3], moderately differentiated OSCC [Table/Fig-4] and poorly differentiated OSCC [Table/Fig-5], the mean BVD was found to be 100.05, 147.13, 151.48, and 159.37 respectively [Table/Fig-6] . Kruskal-wallis test (ANOVA) was applied on the results to compare the BVD between normal oral mucosa and different grades of OSCC and p-value was found to be significant (p<0.05)
The inter-comparison results were assessed by Mann-Whitney U-test. P-value obtained was also significant (<0.05) in all the inter comparisons i.e. normal oral mucosa vs well differentiated OSCC, well differentiated OSCC vs moderately differentiated OSCC, moderately differentiated OSCC vs poorly differentiated OSCC. Therefore, the number of blood vessels increased in transition from well differentiated OSCC to moderately differentiated OSCC and from moderately differentiated OSCC to poorly differentiated OSCC.
Mast Cell Density (MCD ): Mast cells appeared purplish in colour with Toluidine blue stain. Mast cells were counted in the same way and on the same fields as that for blood vessels. When the number of mast cells were evaluated in normal oral mucosa [Table/Fig-2] , well differentiated OSCC [Table/Fig-3] , moderately differentiated OSCC [Table/Fig-4] and poorly differentiated OSCC [Table/Fig-5], the mean MCD was found to be 32.85, 43.75, 50.48, and 50 respectively [Table/Fig-7] . Kruskal-wallis test (ANOVA) was applied on the results to compare the number of mast cells in normal oral mucosa and different grades of OSCC, p-value was found to be significant in well differentiated OSCC (p <0.05).
The inter-comparison results were subjected to Mann-Whitney U test. P-value was found to be significant in the inter-comparison between normal oral mucosa vs well differentiated OSCC, normal oral mucosa vs moderately differentiated OSCC, normal oral mucosa vs poorly differentiated OSCC (p <0.05), and not significant in well differentiated OSCC vs moderately differentiated OSCC and moderately differentiated OSCC vs poorly differentiated OSCC(p >0.05).
Hence, the number of mast cells increased in different grades of OSCC with significant increase in well differentiated OSCC.
Finally, from the above statistical tests it was found that both the variables i.e BVD and MCD were correlated in different grades of OSCC with significant correlation in well-differentiated OSCC [Table/Fig-8] .
Discussion
A tumour growth always requires cell proliferation over cell death or apoptosis. After a growth up to certain millimeters, tumour angiogenesis helps in further tumour growth. It has been shown that, with increased vascular response there is decrease in tumour cell apoptosis, levels of tumour cell proliferation remains constant, leading to net tumour growth [17]. The process of angiogenesis is the outcome of an imbalance between positive and negative angiogenic factors produced by both tumor and host cells [18]. Among the host immune cells several studies have indicated that mast cells play an important role in promoting angiogenesis in some carcinomas like gastric carcinoma, melanoma, esophageal carcinoma, etc [19,20], but very little of its role is known in OSCC. An experimental result suggests that tumour growth can be stopped by various agents that have the capability to inhibit angiogenesis. Therefore, many studies are focused on finding the factors responsible for this very complex process i.e. angiogenesis.
Mast cells release various angiogenic factors that directly or indirectly promote tumour angiogenesis [21]. Tryptase is a serine endopeptidase that is released in abundant quantities from the mast cells in a bound form with heparin. Both the tryptase and heparin are potent angiogenic factors [22]. Also, the basic FGF, IL 4, IL 8, TNF-α, TNF-β are among the mediators released by the granules of mast cells which are strong inducers of angiogenesis [23,24].
Iamaroon et al., studied micro vessel and mast cell density in normal oral mucosa, different grades of dysplasia and OSCC [25]. The densities of micro vessels and mast cells appeared to increase with disease progression. Their findings suggest mast cells may upregulate the angiogenesis in OSCC.
Kalra et al., studied blood vessel density and mast cell density in different histopathological grades of OSCC [26]. Significant increase in blood vessel density was noted in all the grades of OSCC, whereas, mast cell density was decreased from well, moderate to poorly differentiated OSCC. A significant but inverse correlation was seen between blood vessel density and mast cell density in their study.
Evaluation of blood vessels has been done using several vascular markers in recent studies. The most commonly used markers were VEGF, CD105, CD31, CD34 and von Willebrand factor (vWF) [27]. Of these, CD105, CD34 and vWF have the tendency to stain small, large and newly formed blood vessels. CD31 stains only large vessels sometimes tumour cells as well. CD105 doesn’t stain mature vessels. vWF stains lymph vessels too [28]. Thus considering the above disadvantages of the markers, frequency of using CD34 in laboratories and ease of the procedure, we used CD34 marker for blood cell evaluation.
Evaluation of mast cells is best done using Toluidine blue stain. In our study, the slide stained with the CD34 marker was counterstained with Toluidine blue to count blood vessels and mast cells in the same section. Most of the reports measure mast cells and blood vessels in different optical fields and sections making their correlation not warranted. Therefore, this counterstaining technique was adopted to remove technical errors [15].
In the present study, BVD was significantly increased in different grades of OSCC as compared to normal oral mucosa. Therefore, the angiogenic switch seems to be turned on in epithelial malignant transformation. These findings were consistent with other reports in the literature [15,16], stressing the need for research on tissue alterations resulting in increased early angiogenic activity, which through various mechanisms causes epithelial malignant transformation.
Also, MCD was found to be increased in different grades of OSCC as compared to normal oral mucosa, with significant increase in well-differentiated OSCC. MCD in moderately differentiated and poorly differentiated OSCC was almost same. The reason for this could be due to the limited sample size of poorly differentiated OSCC. BVD and MCD were correlated in different grades of OSCC with significant correlation found in well differentiated OSCC. This means that, mast cells are one of the important angiogenic factors promoting tumour angiogenesis. But it is to be noted that in our study, though there was significant increase in mast cell density in well differentiated squamous cell carcinoma, there was not much difference in the MCD between well and poorly differentiated squamous cell carcinoma, this suggests that as grade of tumour increases other angiogenic factors may play a more significant role in tumour angiogenesis [16].
Our findings were similar to the study done Michailidou EZ et al., [15]. They studied mast cells and angiogenesis in oral malignant and premalignant lesions using the same technique. In their study, micro vessel density (MVD) was consecutively increased from normal oral mucosa to leukoplakia to OSCC. Mast cell density (MCD) was also consecutively increased from normal oral mucosa to dysplasia to OSCC. Thus, MCD and MVD were significantly correlated in their study.
Pyogenic granuloma showing CD34 positive blood vessels (10X)
Pyogenic granuloma showing mast cells stained (purple) with Toluidine blue stain(10X)
Light microscopic view of CD34 positive blood vessels and mast cells stained by Toluidine blue in normal oral mucosa (40X) {Light yellow labels shows number of CD34 positive blood vessels in the section, Light blue labels shows number of purple coloured mast cells in the section, The approx. 40,000μm2 is the area of the given section under 40X magnification}
Light microscopic view of CD34 positive blood vessels and mast cells stained by Toluidine blue in well differentiated oral squamous cell carcinoma (40X) {Light yellow labels shows number of CD34 positive blood vessels in the section, Light blue labels shows number of purple coloured mast cells in the section, The approx. 40,000μm2 is the area of the given section under 40X magnification}
Light microscopic view of CD34 positive blood vessels and mast cells stained by Toluidine blue in moderately differentiated oral squamous cell carcinoma (40X) {Light yellow labels shows number of CD34 positive blood vessels in the section, Light blue labels shows number of purple coloured mast cells in the section, The approx. 40,000μm2 is the area of the given section under 40X magnification}
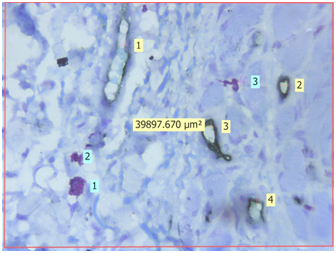
Light microscopic view of CD34 positive blood vessels and mast cells stained by Toluidine blue in poorly differentiated oral squamous cell carcinoma (40X) {Light yellow labels shows number of CD34 positive blood vessels in the section, Light blue labels shows number of purple coloured mast cells in the section, The approx. 40,000μm2 is the area of the given section under 40X magnification}
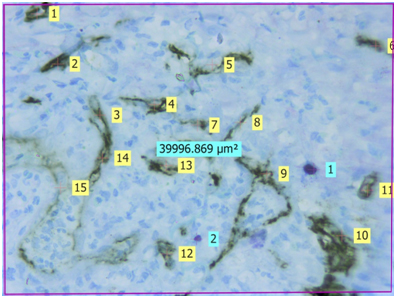
Table showing mean BVD values of normal oral mucosa and different grades of oral squamous cell carcinoma
| Tissue | N | Mean BVD | (p-value) |
|---|
| Normal Mucosa | 10 | 100.05 | <0.05 |
| Well differentiated OSCC | 11 | 147.13 |
| Moderately differentiated OSCC | 13 | 151.48 |
| Poorly differentiated OSCC | 4 | 159.37 |
Table showing mean MCD values of normal oral mucosa and different grades of oral squamous cell carcinoma
| Tissue | N | Mean MCD | (p-value) |
|---|
| Normal Mucosa | 10 | 32.85 | <0.05 |
| Well differentiated OSCC | 11 | 43.75 |
| Moderately differentiated OSCC | 13 | 50.48 | >0.05 |
| Poorly differentiated OSCC | 4 | 50 |
Table showing correlation between mean MCD and BVD values between different grades of oral squamous cell carcinoma
| Tissue | N | Mean MCD | Mean BVD | Correlation between Mean BVD and Mean MCD(p-value) |
|---|
| Normal Mucosa | 10 | 32.85 | 100.05 | <0.05 |
| Well differentiated OSCC | 11 | 43.75 | 147.13 |
| Moderately differentiated OSCC | 13 | 50.48 | 151.48 | >0.05 |
| Poorly differentiated OSCC | 4 | 50 | 159.37 |
Conclusion
From our study, we conclude, that mast cells are one of the major angiogenic factors responsible for promoting tumour angiogenesis. Mast cells are known to promote angiogenesis and as such have been shown to play a major role in tumour progression. In our study it was found, that though mast cells may play an important role in the initial stages of tumour progression, as the grade of the tumour increases, other angiogenic factors secreted by either oncogenic cells, stromal cells or host immune cells like VEGF, FGF, TGF-β may play a more significant role than the mast cells.
List of Abbreviations
OSCC- Oral Squamous cell carcinoma
MCD- Mast cell density
BVD- Blood vessel density
VEGF- Vascular endothelial growth factor
MVD- Micro vessel density
vWF- von Willebrand factor
FGF- Fibroblast growth factor
IL 6- Interleukin 6
IL 8- Interleukin 8
TNFα- Tumour necrosis factor alpha
TNFβ- Tumour necrosis factor beta
APES- 3- Aminopropyl triethoxysilane
DPX- Dibutylphthylate xylol
TGF-β- Transforming growth factor beta
PF4- Platelet factor 4
TSP 1- Thrombospondin 1
[1]. JP Shah, B Singh, Keynote comment: why the lack of progress for oral cancer?Lancet Oncol 2006 15:356-57. [Google Scholar]
[2]. EA Omar, The Outline of Prognosis and New Advances in Diagnosis of Oral Squamous Cell Carcinoma (OSCC)Journal of Oral Oncol 2013 190:1-3. [Google Scholar]
[3]. JK Field, Oncogenes and tumour–suppressor genes in squamous cell carcinoma of the head and neckEur J Cancer B Oral Oncol 1992 28B:67-76. [Google Scholar]
[4]. L Lo Muzio, A Santarelli, V Panzarellan, Oral squamous cell carcinoma and biological markers: an update on the molecules mainly involved in oral carcinogenesisMinerva Stomatol 2007 56(6):341-47. [Google Scholar]
[5]. K Tae, AK El-Naggar, E Yoo, Expression of vascular endothelial growth factor and micro vessel density in head and neck tumourigenesisClin Cancer Res 2000 6:2821-28. [Google Scholar]
[6]. HH Lichtenbeld, MC van Dam-Mieras, HF Hillen, Tumour angiogenesis: pathophysiology and clinical significanceNeth J Med 1996 49:42-51. [Google Scholar]
[7]. KJ Kim, B Li, J Winer, Inhibition of vascular endothelial growth factorinduced angiogenesis suppresses tumour growth in vivoNature 1993 362:841-44. [Google Scholar]
[8]. D Mukhopadhyay, L Tsiokas, XM Zhou, D Foster, JS Brugge, VP Sukhatme, Hypoxic induction of human vascular endothelial growth factor expression through c-Src activationNature 1995 375:577-81. [Google Scholar]
[9]. JL Arbiser, MA Moses, CA Fernandezm, Oncogenic H-ras stimulates tumor angiogenesis by two distinct pathwaysProc Natl Acad Sci U S A 1997 94:861-66. [Google Scholar]
[10]. M Tomita, Y Matsuzaki, M Edagawa, T Shimizu, M Hara, R Sekiya, Association of the mast cells with the tumor angiogenesis in esophageal squamous cell carcinomaDis Esophagus 2001 14:135-38. [Google Scholar]
[11]. DD Metcalfe, D Baram, YA Mekori, Mast cellsPhysiol Rev 1997 77(4):1033-79. [Google Scholar]
[12]. Y Hiromatsu, S Toda, Mast cells and angiogenesisMicrosc Res Tec 2003 60:64-69. [Google Scholar]
[13]. HH Oliveira-Neto, AF Leite, NL Costa, Decrease in mast cells in oral squamous cell carcinoma: Possible failure in the migration of these cellsOral Oncol 2007 43:484-90. [Google Scholar]
[14]. NL Costa, AF Oton-Leite, AP Cheim-Junior, Density and migration of mast cells in lip squamous cell carcinoma and actinic cheilitisHistol Histopathol 2009 24:457-65. [Google Scholar]
[15]. EZ Michailidou, AK Markopoulos, DZ Antoniadesn, Mast cells and angiogenesis in oral malignant and premalignant lesionsOpen Dent J 2008 28:126-32. [Google Scholar]
[16]. M Macluskey, LM Chandrachud, S Pazouki, Apoptosis, proliferation, and angiogenesis in oral tissues: Possible relevance to tumour progressionJ Pathol 2000 191:368-75. [Google Scholar]
[17]. G Jahanshahi, M Sabaghian, Comparative immunohistochemical analysis of angiogenesis and mast cell density in oral normal mucosa and squamous cell carcinomaDental Research Journal 2012 9(1):8-12. [Google Scholar]
[18]. S Pazouki, DS Chisholm, MM Adi, The association between tumour progression and vascularity in the oral mucosa J Pathol 1997 183:39-43. [Google Scholar]
[19]. D Ribatti, D Guidoli, A Marzullo, Mast cells and angiogenesis in gastric carcinomaInt. J. Exp. Path 2010 91:350-56. [Google Scholar]
[20]. GÖ Elpek, T Gelen, NH Aksoy, The prognostic relevance of angiogenesis and mast cells in squamous cell carcinoma of the oesophagusJ Clin Pathol 2001 54:940-44. [Google Scholar]
[21]. BL Gruber, MJ Marchese, R Kew, Angiogenic factors stimulate mast-cell migrationBlood 1995 86:2488-93. [Google Scholar]
[22]. LB Schwartz, Tryptase: A mast cell serine proteaseMet Enzymol 1994 244:88-100. [Google Scholar]
[23]. JR Gordon, SJ Galli, Promotion of mouse fibroblast collagen gene expression by mast cells stimulated via the Fc epsilon RI. Role for mast cell-derived transforming growth factor beta and tumor necrosis factor alphaJ Exp Med 1994 180:2027-37. [Google Scholar]
[24]. Y Okayama, A Semper, ST Holgate, MK Church, Multiple cytokine mRNA expression in human mast cells stimulated via Fc epsilon RIInt Arch Allergy Immunol 1995 107:158-59. [Google Scholar]
[25]. A Iamaroon, S Pongsiriwet, S Jittidecharaks, K Pattanaporn, S Prapayasatok, S Wanachantararak, Increase of mast cells and tumor angiogenesis in oral squamous cell carcinomaJ Oral Pathol Med 2003 32:195-99. [Google Scholar]
[26]. M Kalra, N Rao, K Mehta, The role of mast cells on angiogenesis in oral squamous cell carcinomaMed Oral Patol Oral Cir Bucal 2012 17(2):e190-96. [Google Scholar]
[27]. Z Jaafari Ashkavandi, M Moshref, F Mashhadi-Abbas, A Sargolzaie, N Taghavi, Evaluation of CD31 expression and mast cell count in dysplastic lesions and squamous cell carcinoma of the oral cavityJ Iran Red Crescent Med 2010 12:272-76. [Google Scholar]
[28]. M Moriyama, S Kumagai, S Kawashiri, K Kojima, K Kakihara, E Yamamoto, Immunohistochemical study of tumor angiogenesis in oral squamous cell carcinomaOral Oncol 1997 33:369-74. [Google Scholar]