Introduction
Glass Ionomer cements (GIC’s) are clinically attractive dental materials with unique properties that make them useful restorative materials which include chemical adhesion, anticariogenic properties, thermal compatibility, biocompatibility and low toxicity [1]. The caries-inhibitor influence of GIC sufficient to completely arrest the caries process is doubtful and clinical studies show that residual bacteria located under a GIC restoration are viable for up to two years [2]. The use of GICs in a mechanically loaded situation, however, has been hampered by their low mechanical properties thus limiting their use in stress bearing areas [3]. In this concept, the current research was designed to study the influence of addition of hydroxyapatite to GIC on its compressive strength and antibacterial activity when immersed in different storage media.
Materials and Methods
The present study was carried out at Bapuji dental college and hospital in Department of Pedodontics and Preventive Dentistry between the academic years 2010 to 2013. The materials used in study were selected based on the following criteria. GC Fuji Type IX gold label (GC corporation, Tokyo, Japan) glass ionomer was selected in the study since glass ionomer is the most commonly used restorative material for posterior teeth in pediatric patients because of its various advantageous properties like fluoride release, chemical adhesion and type IX is high strength GIC most commonly used for ART technique. Synthetic Hydroxyapatite bone graft for dental surgery-ASTM F 1185 03 (SYBOGRAF TM) was used since it is proven through various studies that incorporation of hydroxyapatite results in increase in mechanical properties apart from increasing antibacterial activity. Test solutions like deionized water, artificial saliva and lactic acid were selected in order to simulate oral condition. The antibacterial activity was evaluated against Streptococcus mutans (ATCC no 25175) since it is the main organism which is responsible for the causation of dental caries.
Preparation of Test Specimen
A total of 126 cylindrical test specimens of dimensions 4 mm diameter x 6 mm height were prepared from a custom made brass mould using the following restorative test materials. Of 126 test specimens, 63 of them were used to prepare specimens for conventional GIC material which served as the experimental group. A thin layer of petroleum jelly was coated on the lateral walls of the moulds to prevent material adhesion. The powder and liquid of conventional GIC were mixed according to manufacturer’s instructions. The mixed cement was placed into the custom made brass mould [4] by slightly overfilling them and was placed between two glass plates with the mylar strips placed between brass mould, a glass plate was used to prevent the adhesion of GIC to glass slab. The glass plates were held firmly during setting to avoid the presence of air bubble and to obtain a smooth surface. After setting, the pellets were removed from the mould and the excess was trimmed using a Bard Parker blade, the specimens were stored in deionized water at temperature of 37°C [5]. Commercially available Hydroxyapatite powder (SYBOGRAF TM) was used in the study. Eight percent by weight of Hydroxyapatite powder was mixed with powder of conventional GIC and this Hydroxyapatite added cement was mixed with polyacrylic acid liquid of conventional GIC [6]. Sixty three test specimens were prepared as mentioned previously and stored in deionized water at 37°C.
Preparation of Artificial Saliva
It was prepared in the laboratory from 0.4gms sodium chloride (NACL), 1.21 gms potassium chloride (KCL), 0.78 gms sodium dihydrogen dehydrate (NaH2PO4.2H2O), 0.005 gms hydrated sodium sulfide (Na2S.9H2O), 1gms urea CO(NH2)2 and 1000 ml of deionized water. 10N sodium hydroxide was added to this mixture until the pH value was measured to be as 6.75±0.15. Later the mixture was sterilized in the autoclave [7].
Assessment of Compressive Strength
Conventional glass ionomer cement (GC Fuji IX gold label) served as control. Hydroxyapatite was added to glass ionomer cement at concentration ratios of 8% w/w to obtain the experimental groups [6].
One hundred and twenty six pellets of conventional GIC and hydroxyapatite added to GIC were prepared of the specific dimension of 6 x 4mm and kept in deionized water at a temperature of 37°C. One hundred and twenty six pellets divided were equally into 6 groups [Table/Fig-1].
All the control and experimental groups were kept under deionized water, artificial saliva, lactic acid solution respectively for 3 hours everyday over 30 days test period. The test solutions were changed with fresh solution every week for 30 days. The compressive strength of each material was measured by using a universal testing machine (AG-50kNG) at cross head of 1mm2/min and strength was determined after 1 day, 7 days and 30 days respectively [8].
Assessment of Antibacterial Activity
Total of 16 specimens of conventional GIC and hydroxyapatite added to GIC each were used to check the antibacterial activity against Streptococcus mutans strain. The microbial strains were obtained from the American Type Culture Collection (ATCC), USA. Nutrient broth was used to get the viable growth of microbes from freeze dried form. Turbidity in test tube confirmed the growth of microbes. Comparison of this turbidity was made with McFarland 0.5 turbidity standard which was prepared by mixing 0.05mL of 1.175% barium chloride dihydrate (BaCl2•2H2O), with 9.95mL of 1% sulfuric acid (H2SO4).
Serial dilution technique was used to check the antibacterial activity of GIC against Streptococcus mutans. Ten tubes were arranged serially. In the 1st tube 400microlitre of BHI broth was added. For further dilutions, 200 microliter of BHI broth was added into the next 9 tubes separately. The pellet samples were added to this first tube and allowed to stand for few minutes. Then from the initial tube, 200 micro liters was transferred to the second tube containing 200 microliter of BHI broth. This was considered as 10-1 dilution. From this 10-1 diluted tube, 200 microliter was transferred to second tube to make 10-2 dilution. The serial dilution was repeated up to 10-9 dilution for each drug. From the maintained stock culture of Streptococcus mutans, 5microliter was taken and added into 2ml of BHI (brain heart infusion) broth. In each serially diluted tube 200 microliter of above culture suspension was added. The tubes were incubated for 16-18 hours and observed for turbidity. First four tubes were inoculated on to the BHI agar plates and incubated for 24 hours and checked for the presence or absence of the growth. Inhibitions of bacterial growth were assessed by enumerating the number of colonies in each section using colony counter [9].
Statistical Analysis
The results were tabulated and statistically analysed with Intragroup comparisons made by one-way ANOVA followed by post-hoc Tukey’s test. Intergroup comparison was done with Mann-Whitney test. A p-value of 0.05 or less was considered as statistically significant.
Results
[Table/Fig-2] shows mean, standard deviation and Intragroup comparison of the compressive strength of GIC immersed in water, artificial saliva and lactic acid after 24 hours, 7 days and 30 days. Groupwise comparison of compressive strength of different groups (Group 1,2 and 3) at 24 hours, 7 days and 30 days showed statistical significance among these group (p<0.01) with mean compressive strength of Group 2 (GIC immersed in artificial saliva) showing highest value among all the groups compared.
[Table/Fig-3] shows mean, standard deviation and intragroup comparison of the compressive strength of GIC immersed in water, artificial saliva and lactic acid after 24 hours, 7 days and 30 days. Groupwise comparison of compressive strength of different groups (Group 4,5 and 6) at 24 hours, 7 days and 30 days showed statistical significance among these group (p<0.01) with mean compressive strength of Group 5 (GIC±HAp immersed in artificial saliva) showing highest value among all the group compared.
[Table/Fig-4] shows mean, standard deviation and intergroup comparison of the compressive strength of GIC and GIC±HAp immersed in water, artificial saliva and lactic acid after 24 hours, 7 days and 30 days. Intergroup comparison of compressive strength at different time interval showed no statistical significance except in the lactic acid media (Group 3 and Group 6) which showed statistical significance with p-value 0.03 at the end of 24 hours.
[Table/Fig-5] shows mean, standard deviation, median and range of the antibacterial activity of GIC and GIC±HAp measured at 16-18 hours. The mean colony count for GIC was 371.3±185.7 and GIC±HAp were 209.9±181.5 with median and range of GIC and GIC±HAp as 500 and 142, 58-550 and 0-500. The intergroup comparison showed GIC±HA p-values were statistical significant than conventional GIC (p-value is 0.005).
Discussion
Dental caries is ubiquitous and is one of the most prevalent infectious diseases of man. The demineralization is caused by acids produced by bacteria, particularly Streptococcus mutans that ferments dietary carbohydrates [10].
In this era of technological advancements many dental materials are available for the treatment of dental caries. The fluoride releasing restorative materials have gained wide popularity in treatment of dental caries because of its cariostatic property. One such material which was most commonly used in pediatric dentistry is GIC.
The last few decades have witnessed efforts increasingly directed towards the discovery and development of newer dental materials and the modification of the older materials with the addition of various materials which were proven to be beneficial. One such modification made was by incorporation of hydroxyapatite to the glass ionomer cement.
Hydroxyapatite (HAp) has shown promising advantages in restorative dentistry, including its biocompatibility, hardness similar to that of natural tooth and intrinsic radiopaque response. Various reinforcement materials such as HAp had shown high success when added to either composite or adhesive bone cements [6]. Studies have reported that when HAp is added to cement it helps in improving mechanical property such as surface hardness, toughness, flexural strength and modulus [11,12].
Kenji Arita concluded that the highest increase in the flexural strength of the GIC cement was achieved with the addition of 8% HA granules in their study [6]. Hence, to check for the influence of same concentration of hydroxyapatite on the compressive strength of GIC, it was used in present study.
Various storage media were used in this study in order to simulate oral condition. The ability of restorative dental materials to withstand Acidthe functional force and exposure to various media in the mouth is an important requirement for their clinical performance for a considerable period of time.
Specific dimension of the brass mould was selected based on the ISO specification (7489: 1986) and according to British Standard (BS 6039: 1981), where specimens are defined to have a height of 6 mm and a diameter of 4 mm.
Mallmann A suggested the use of smaller specimen dimension (6 x 4mm) since the glass ionomer material are moisture sensitive which is subjected to change in properties during the manual manipulation of cement depending on temperature and moisture contamination [4].
The first objective of study was designed to evaluate and compare the compressive strength of GIC and 8% HAp added to GIC immersed in storage media i.e. deionized water, artificial saliva and lactic acid checked at different time intervals.
The observation in the present study indicates that conventional GIC and 8% hydroxyapatite added GIC when immersed in the different storage media had significant changes in compressive strength of material. There was a marked increase in compressive strength of groups in artificial saliva when compared with that of the groups deionized water and groups lactic acid.
Similar observations made by K Okada et al., who evaluated increased in the surface hardness of GIC when stored in saliva; it was probably the result of salivary components including calcium and phosphate diffusing into the cement structure [13].
Another possible reason for increased strength may be Ca ion, which exists in saliva and not in Fuji IX, also would have a coordination number of six. It might diffuse into the matrix and have the ligands which might be carboxylate groups of polyacrylic acid, phosphorus ion in saliva and water molecules. Thus, increasing the strength of the material [13].
The compressive strength in acidic media showed less when compared to neutral solution because in acidic media, various ions like aluminum, silicon and phosphorus, like calcium, showed increased released with time. This suggests an increase in the proportion of acid-soluble components with time, which in turn suggests that the change is a consequence of the maturation processes thus reducing the final structure of the set cement which future effect strength of the cement [14].
These improved properties are thought to be:
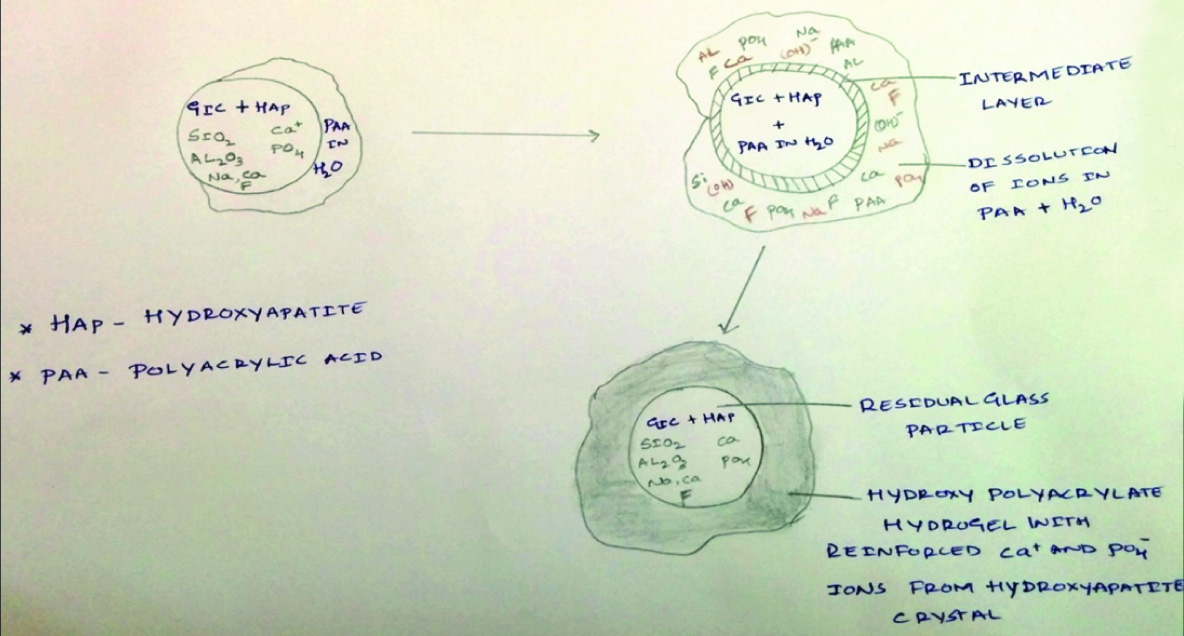
1. The matrix of HAp-added GIC was strengthened compared to conventional GIC by increased metal ions participating in the cement hardening reaction. The metal ions (Ca2±) are supplied by the dissolution of HAp particles.
2. The HAp particles in GIC was reinforced first by the adsorption of GIC matrix and then by the formation of an “intermediate layer” between the surface of the primary HAp crystals and the absorbed matrix.
3. HAp-added GIC was reinforced by adhesion of the improved matrix and the improved HAp particles [Table/Fig-6].
On the contrary, Yamamoto and Nicholson reported that the compressive strength of HAp containing GIC decreased as the mass of HAp increased. This observation may be attributed to the greater volume of HAp used, which is softer than the glass particles. Other reasons may also be insufficient polyacrylic acid to hydrolyze the HAIonomer powder mixture effectively leading to inadequate matrix formation and decrease resistance to indentation. The decrease in compressive strength could be attributed to the time-dependent dissolution of HAp[15].
Goenka S evaluated the mechanical properties of GIC (Fuji IX) with fluorhydroxyapatite nanoparticles and assessed for the influence of the particles on the compressive strength, hardness, and fracture toughness. The results concluded that significant increase in hardness with little or no significant change was found for fracture toughness or compressive strength even though it appeared to be increased in compressive strength with increase in wt percent of nanoparticles to cement [16]
Moshaverinia et al., incorporated synthesized nanoceramic (nanohydroxy and fluoroapatite) particles which were incorporated into commercial glass ionomer powder (Fuji II GC) and evaluated for compressive, diametral tensile and biaxial flexural strengths and bond strength to dentin. The results concluded that there was significant increase in the compressive diametral tensile and biaxial flexural strengths when compared to control group after period of 7days.Bond strength to dentin increased significantly over period of 30days [17].
Mallmann et al., compared two specimen dimension (One with 6 mm in height and 4 mm in diameter and the other with 12 mm in height and 6 mm in diameter, according to ISO 7489:1986 specification and the ANSI/ADA Specification No. 66 for Dental GlassIonomer Cement, respectively) on compressive strength of two commercially available GIC cements. The results of the study concluded that for both glass ionomer materials, the 12 mm x 6 mm matrix led to higher compressive strength results compared to the 6 mm x 4 mm matrix [18].
After extensive exploration of literature, it was revealed no studies exist which compared antibacterial activity of hydroxyapatite added to conventional GIC. As HAp is not readily dissolved in distilled water or saline, the assessment of microbial growth inhibition using the agar-diffusion test was not a suitable option. Therefore, to measure antibacterial activity broth dilution technique was used in the present study [19].
The observation made from the present study indicates the conventional GIC had shown less antibacterial activity when compared to that of the hydroxyapatite added to conventional GIC.
Tin-Oo M conducted a study to assess the antibacterial property of locally produced hydroxyapatite against S mutans and concluded the antibacterial activity depends on the concentration of hydroxyapatite. This study reiterates the fact that HAp possesses antibacterial activity as evinced by Ingram et al., and Owadally et al., [19].
Zalewska A conducted a study to evaluate antibacterial activity of different types of GIC {Fuji Triage (GC), Fuji IX (GC), Ketac Molar (3M ESPE) and Ketac Silver (3M ESPE)} against various cariogenic bacteria which includes Streptococcus mutans, Streptococcuss anguis, Streptococcuss alivarius and Lactobacillus casei and concluded that Fuji IX GIC showed potent antibacterial activity against Streptococcussanguis but with overall antibacterial activity Fuji triage showed most potent and Fuji IX showed least activity [20].
Showing group distribution of control and experimental group
| Groups |
|---|
| I | Conventional GIC immersed in water |
| II | Conventional GIC in artificial saliva |
| III | Conventional GIC in lactic acid |
| IV | Conventional GIC+HAp immersed in water |
| V | GIC+HAp in artificial saliva |
| VI | GIC+HAp in lactic acid |
Descriptive Data and Intragroup Comparison for the Compressive Strength of Gic in Various Media at 24 Hours, 7 Days and 30 Days (In Mpa).
| GIC subgroups | 24 Hours | 7 Days | 30 Days |
|---|
| Deionized water | 113±3.4 | 126.2±2.3 | 129.8±2.5 |
| Artificial saliva | 120±3.5 | 134.7±2.8 | 135.5±2.8 |
| Lactic acid | 106.9±3.0 | 120.4±2.1 | 121.4±3.3 |
| ANOVA F | 28.74 | 61.35 | 41.52 |
| P | <0.001 | <0.001 | <0.001 |
| ***Intragroup Comparision (p-Values) | 1-2 | <0.01 | <0.01 | 0.005 |
| 1-3 | 0.005 | 0.001 | 0.00 |
| 2-3 | <0.01 | <0.01 | <0.01 |
*Intragroup Comparision (p-Values)
* one-way anova test followed by post-hoc ‘t’ test
Descriptive data and intragroup comparison for the compressive strength of GIC+HAp in various media At 24 hours, 7 days and 30 days (In Mpapa)
| GIC+HA p subgroups | 24 Hours | 7 Days | 30 Days |
|---|
| Deionized water | 112.5±3.9 | 127.1±3.8 | 128.4±3.3 |
| Artificial saliva | 122.5±3.5 | 134.5±2.3 | 135.2±3.3 |
| Lactic acid | 111.9±4.3 | 120.0±1.6 | 121.4±2.8 |
| ANOVA F p | 16.21 <0.001 | 49.02 <0.001 | 33.61 <0.001 |
| *INTRAGROUP COMPARISION (p-VALUES) | 4-5 | <0.01 | <0.01 | 0.002 |
| 4-6 | 0.96 | 0.00 | 0.003 |
| 5-6 | <0.01 | <0.01 | <0.01 |
*Intragroup Comparision (p-Values)
* one-way anova test followed by post-hoc ‘t’ test
Intergroup Comparison of Gic and Gic +HAp in Various Media At 24hrs, 7days and 30days (In Mpa )
| Time of Assessment | Different media | GIC | GIC+HA p | GIC v/s GI C+HA p |
|---|
| Mean diff | t-value | p-value |
|---|
| 24HRS | DEIONIZED WATER | 113.3±3.4 | 112.5±3.9 | 1.2 | 0.64 | 0.53 |
| ARTIFICIAL SALIVA | 120±3.5 | 122.5±3.5 | 2.5 | 1.35 | 0.20 |
| LACTIC ACID | 106.9±3.0 | 111.9±4.3 | 5.0 | 2.54 | 0.03 |
| 7DAYS | DEIONIZED WATER | 126.2±2.3 | 127.1±3.8 | 0.9 | 0.53 | 0.61 |
| ARTIFICIAL SALIVA | 134.7±2.8 | 134.5±2.3 | 0.2 | 0.10 | 0.92 |
| LACTIC ACID | 120.4±2.1 | 120.0±1.6 | 0.4 | 0.47 | 0.65 |
| 30DAYS | DEIONIZED WATER | 129.8±2.5 | 128.4±3.3 | 1.4 | 0.83 | 0.42 |
| ARTIFICIAL SALIVA | 135.5±2.8 | 135.2±3.3 | 0.3 | 0.14 | 0.89 |
| LACTIC ACID | 121.4±3.3 | 121.4±2.8 | 0.0 | 0.00 | 1.00 |
Intergroup Comparison of the Antibacterial Activity of Gic and Gic +HAp at 16-18 Hours (In Cfu)
| Mean±SD | Median | Range |
|---|
| GIC | 371.3±185.7 | 500 | 58-550 |
| GIC+HAp | 209.9±181.5 | 142 | 0-500 |
| GIC V/S GIC+HAp | * p-VALUE= 0.005 |
*Mann-Whitney Test
Representing Setting Reaction of Gic Reinforced HAp with Polyacrylic Acid
Limitation of the Study
The present study considered only 8% of hydroxyapatite incorporated into GIC, effect of different concentration of hydroxyapatite on GIC can be considered.
The study included only one specimen dimension (6X4mm) but the effect of different specimen dimension on compressive strength can be studied.
Only compressive strength was included in study since the influence of this cement on other mechanical property have to be studied.
Only Streptococcus mutans strain was used to assess antibacterial activity but lactobacillus can also be considered.
Clinical Application
The main disadvantage of GIC is its lack of strength, because of which it is contraindicated in high stress bearing areas, incorporation of hydroxyapatite crystals into GIC not only improved its mechanical properties but also antibacterial activity which extends its application in restoration of high stress bearing areas. The improved antibacterial activity combats secondary caries and aids in patient with high caries risk.
Conclusion
Based on the observation of the present study, it can be concluded that by addition of 8% hydroxyapatite to conventional GIC has improved its antibacterial activity when compared to that of the conventional GIC. Addition of 8% hydroxyapatite to conventional GIC showed no significant changes in the compressive strength when immersed in the different storage media except for the increase in compressive strength when immersed in the lactic acid. Addition of 8% hydroxyapatite to conventional GIC showed no significant changes in the compressive strength when assessed at different time intervals.
Acknowledgements
The authors would like to acknowledge all the staff for their support in making this study success.
*Intragroup Comparision (p-Values)* one-way anova test followed by post-hoc ‘t’ test
*Intragroup Comparision (p-Values)* one-way anova test followed by post-hoc ‘t’ test
*Mann-Whitney Test
[1]. GJ Mountn, Glass ionomers: a review of their current statusOper Dent 1999 24(2):115-24. [Google Scholar]
[2]. C Yesilyurt, K Er, T Tasdemir, K Buruk, D Celik, Antibacterial activity and physical properties of glass-ionomer cements containing antibioticsOper Dent 2009 34(1):18-23. [Google Scholar]
[3]. BS Lim, HJ Moon, KW Baek, SH Hahn, CW Kim, Color stability of glass ionomers and polyacid-modified resin-based composites in various environmental solutionsAm J Dent 2001 14(4):241-46. [Google Scholar]
[4]. A Mallmann, JC Ataíde, R Amoedo, PV Rocha, LB Jacques, Compressive strength ofglass ionomer cements using different specimen dimensionsBraz Oral Res 2007 21(3):204-08. [Google Scholar]
[5]. JA Williams, RW Billington, Changes in compressive strength glass-ionomer restorative materials with respect to timeJ Oral Rehab 1991 18:163-65. [Google Scholar]
[6]. K Arita, ME Lucas, M Nishino, The effect of adding hydroxyapatite on the flexural strength of glass ionomer cementDent Mater J 2003 22:126-36. [Google Scholar]
[7]. G Beresescu, LC Brezeanu, Effect of Artificial Saliva on the Surface of Roughness of Glass-Ionomer cementsScientific bulletin of the petrumaioruniversity of targumures 2011 8(2):134-36. [Google Scholar]
[8]. Y Kuboki, K Ohgushi, T Fusayama, Collagen biochemistry of the two layers of carious dentinJ Dent Res 1977 56(10):1233-37. [Google Scholar]
[9]. R Schwalbe, L Steele-Moore, C Goodwin, Antimicrobial susceptibility testing protocolsCrc Press 2007 [Google Scholar]
[10]. JDB Featherstonen, The science and practice of caries preventionJ Am Dent Assoc 2000 131:887-99. [Google Scholar]
[11]. ME Lucas, K Arita, M Nishino, Toughness, bonding and fluoride-release properties of hydroxyapatite-added glass ionomer cementBiomaterials 2003 24(21):3787-94. [Google Scholar]
[12]. YB Mu, GX Zang, HC Sun, CK Wang, Effect of nano-hydroxyapatite to glass ionomer cement Hua Xi Kou Qiang Yi XueZaZhi 2007 25(6):544-47. [Google Scholar]
[13]. K Okada, S Tosaki, K Hirota, WR Hume, Surface hardness change of restorative filling materials stored in salivaDent Mater 2001 17(1):34-39. [Google Scholar]
[14]. B Czarnecka, RO Limanowska-Shaw, H Nicholson, Buffering and ion-release by a glass-ionomer cement under near-neutral and acidic conditionsBiomaterials 2002 23(13):2783-88. [Google Scholar]
[15]. JW Nicholson, SJ Hawkins, JE Smith, The incorporation of hydroxyapatite into glass-polyalkenoate (glass-ionomer) cements: a preliminary studyJ Mater Sci Mater Med 1993 4:418-21. [Google Scholar]
[16]. S Goenka, R Balu, TS Sampath Kumar, Effects of nanocrystallinecalciumdecient hydroxyapatite incorporation in glass ionomer cementsJ MechBehav Biomed Mater 2012 7:69-76. [Google Scholar]
[17]. A Moshaverinia, S Ansari, M Moshaverinia, N Roohpour, JA Darr, I Rehman, Effects of incorporation of hydroxyapatite and fluoro apatite nano bioceramics into conventional glass ionomer cements (GIC)ActaBiomater 2008 4(2):432-34. [Google Scholar]
[18]. A Mallmann, JC Ataíde, R Amoedo, PV Rocha, LB Jacques, Compressive strength of glass ionomer cements using different specimen dimensions Braz Oral Res 2007 21(3):204-08. [Google Scholar]
[19]. M Tin-Oo, V Gopalakrishna, AR Samsuddin, KA Al Sahili, O Shamsuria, Antibacterial property of locally produced hydroxyapatiteArchieves of orofacial sciences 2007 2:41-44. [Google Scholar]
[20]. E Łuczaj-Cepowicz, G Marczuk-Koladal, A Zalewska, M Pawińska, K Leszczyńska, Antibacterial activity of selected glass ionomercementPostepyHig Med Dosw 2014 68:23-28. [Google Scholar]