Introduction
Tobacco is the prime environmental cause of death and disease, with smoked tobacco being the most prevalent and harmful tobacco product. Periodontal disease is one of the common inflammatory diseases with a complex etiology and multifactorial in origin [1].
Saliva is the first biological fluid to encounter cigarette smoke. A number of clinical studies have compared the periodontal status of smokers and non-smokers [2-4]. Biomarkers of tobacco exposure are used to confirm the absorption of specific smoke constituents in a quantitative manner. The advantages of using saliva as a diagnostic tool when compared to Gingival crevicular fluid (GCF) or serum is that- a) sampling is rapid, non-invasive and multiple sample collections; b) the process of collection is simple, painless and doesnot require a skilled work force for sampling; c) ideal for mass screening programs; and d) it is safer as it causes minimal threat to the collector from infectious diseases such as HIV and or Hepatitis during handling of the samples [5].
Thiocyanate ion is derived endogenously as a detoxification product of the reaction between cyanide and thiosulfates in the liver. Determinig thiocyanate (SCN) levels in saliva is one of the biochemical test for establishing the incidence or prevalence of tobacco consumption. Studies have reported that SCN can cause neurological, endocrine alterations in the body and also as a factor in delayed wound healing [6-10]. The half life of SCN is reported to be approximately 14 days, that makes it as a reliable biomarker that confirms tobacco consumption characteristics in the population [11]. The salivary thiocyanate concentration in non-smokers is usually between 0.5-2Mm ( 29-116μg/ml) [12]. Hence, a high saliva thiocyanate concentrations can be qualitatively used an indicator for tobacco exposure.
Saliva may constitute a first line defense against oxidative stress and has protective effects against microorganisms, toxins and oxidants. Uric acid is the most important non-enzymatic antioxidant presnt in saliva correlates with plasma uric acid, suggesting that former is imported from plasma. Low levels of uric acid has been reported in smokers [13,14].
Among the other salivary parameters pH is reported to show an immediate transient increase after smoking. The influence of smoking has not been studied extensively. Some studies reported that salivary pH lie at an alkaline level in patients with periodontal disease [15]. Other studies report that pH is comparatively lesser in smokers than non-smokers [16]. There is no study done in the literature to assess and compare periodontal status of tobacco users and non-users in relation to salivary thiocyanate, uric acid and pH. Hence this study was done to assess the salivary thiocyanate, uric acid levels and pH of tobacco users and non-users of age 35-44 years with their periodontal status.
Material and Methods
A cross-sectional institution based study was conducted to assess the periodontal status of tobacco users and non-users and also to compare their salivary thiocyanate levels, uric acid levels and pH. The study population consisted of subjects attending the Department of Public Health Dentistry and Department of Oral Medicine and Radiology in Yenepoya Dental College in the age group of 35-44 years and were selected based on the following inclusion and exclusion criteria. The study protocol was reviewed and ethical clearance was provided by the Ethical Committee of Yenepoya University.
Distribution according to frequency of tobacco usage
| Frequency of tobacco use | Groups | Total |
|---|
| Smokers | Chewers |
|---|
| n | % | n | % | n | % |
|---|
| 2 to 5 times per day | 17 | 56.67 | 13 | 43.3 | 30 | 50 |
| 6 to 10 times per day | 7 | 23.3 | 14 | 46.7 | 21 | 35 |
| 11 to 15 times per day | 4 | 13.3 | 3 | 10 | 7 | 11.67 |
| More than 15 times/ day | 2 | 6.7 | 0 | 0 | 2 | 3.3 |
| Total | 30 | 100 | 30 | 100 | 60 | 100 |
Mean number of healthy sextants, bleeding or higher score, shallow pockets or higher score, deep pockets among various tobacco users and non- tobacco users
| CPI Scores | Tobacco users (mean ± SD) | Non-tobacco users | Kruskal wallis test value | p-value |
|---|
| Smokers | Chewers |
|---|
| Healthy periodontal tissue | 0.06 ± 1.02` | 0.06 ±1.02 | 1.03 ±0.039 | 14.620 | 0.001 |
| Bleeding or higher score | 5.8 ±1.34 | 5.83 ±1.42 | 5.66 ± 1.23 |
| Calculus or higher score | 5.1 ± 1.08 | 5.2 ± 0.14 | 4.63 ± 0.22 |
| Shallow pockets or higher score | 3.43 ± 1.21 | 3.9 ±0.15 | 2.1 ±0.81 |
| Deep pockets | 0.23 ± 0.81 | 0.53 ±0.04 | - |
Mean number of sextants affected with loss of attachement (LOA), by scores among various tobacco users and non-tobacco users
| LOA Scores | Tobacco users (mean ± SD) | Non-tobacco users | Kruskal wallis test value | p-value |
|---|
| Smokers | Chewers |
|---|
| 0-3mm | 3.2 ± 1.13 | 2.93 ±1.20 | 4.6 ±0.89 | 6.826 | 0.033 |
| 4-5mm | 2.3 ±1.25 | 2.13 ±1.36 | 1.26 ± 0.85 |
| 6-8mm | 0.26 ± 0.72 | 0.53 ± 0.80 | 0.06 ± 0.28 |
| 9-11mm | 0.03 ± 0.25 | 0.10 ±0.28 | - |
| 12mm or more | - | 0.03 ±0.25 | - |
| Not recorded | 0.13 ± 0.35 | 0.10 ±0.29 | - |
Salivary thiocyanate level in tobacco users and non users
| Groups | n | Mean thiocyante levels(μg/ml) | Standard Deviation | Minimum level detected(μg/ml) | Maximum level detected(μg/ml) | ANOVA F-value | p-value |
|---|
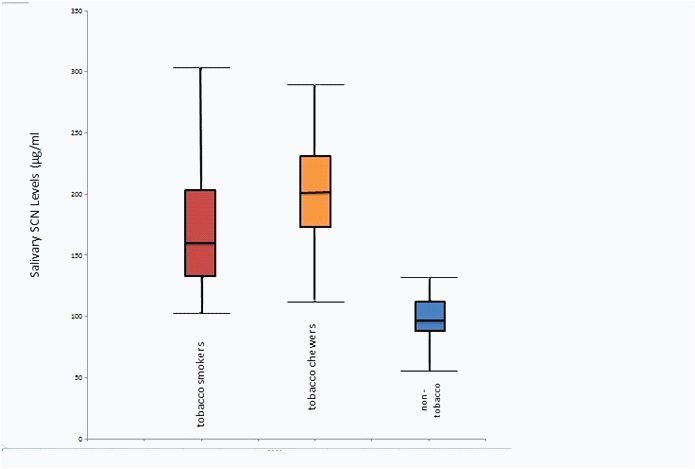
| Smokers | 30 | 172.50 | 54.74 | 103 | 304 | 49.377 | <0.001 |
| Chewers | 30 | 203.70 | 45.77 | 112 | 290 |
| Non-tobacco users | 30 | 97.63 | 17.98 | 56 | 132 |
| Total | 90 | 157.94 | 61.38 | 56 | 304 |
Salivary thiocyanate levels in various tobacco users and non-tobacco users
Salivary uric acid level in various tobacco users and non-tobacco users
| Groups | n | Mean uric acid (mg/dL) | Standard Deviation | Minimum leveldetected (mg/dL) | Maximum level detected(mg/dL) | ANOVA F-value | p-value |
|---|
| Smokers | 30 | 2.54 | 0.63 | 1.15 | 3.84 | 3.301 | 0.045 |
| Chewers | 30 | 2.65 | 0.37 | 1.75 | 3.45 |
| Non-tobacco users | 30 | 2.33 | 0.47 | 1.12 | 2.84 |
| Total | 90 | 2.50 | 0.51 | 1.12 | 3.84 |
Salivary pH in tobacco users and non-tobacco users
| Groups | n | Mean pH | Standard Deviation | Minimum value | Maximum value | ANOVA (F) | p-value |
|---|
| Smokers | 30 | 6.72 | 0.33 | 6.2 | 7.6 | 0.103 | 0.092 |
| Chewers | 30 | 6.76 | 0.46 | 5.5 | 7.8 |
| Non-tobacco users | 30 | 6.76 | 0.32 | 5.5 | 7.6 |
| Total | 90 | 6.75 | 0.37 | 5.5 | 7.8 |
Inclusion criteria
Tobacco smokers: Subjects smoking more than 20 cigarettes/beedi per week for the last one year or more.
Tobacco chewers: Subjects consuming chewable tobacco in any form daily for the last one year or more.
Non-tobacco users: Subjects of same age and sex who haven’t used tobacco in any form in their life time.
Exclusion criteria
Subjects with systemic disease such as cardiovascular problems,malignancies, bronchial asthma, gout and those on long term medication or drugs which may alter the periodontal status.
Subjects who have undergone periodontal therapy within the past six months.
The Periodontal status of the subject were assessed using the Community Periodontal Index and Loss of Attachment scores which are modified form of WHO Community Periodontal Index of Treatment Needs (CPITN). The periodontal experience of Ten subjects who were similar in age to the study subjects were recorded using the same Index. The examiner practised the Index on Ten subjects. The findings were compared with the scores obtained by another faculty member in the Department of Public Health Dentistry.The inter-examiner reliability was assessed and the kappa value was found to be 0.86, reflecting a high degree of agreement in the observations. One of the postgraduate student from the Department was the recorder for the study and he participated in all the training exercises and the examination procedures.
Saliva Collection and Analysis of Parameters
The saliva samples were collected between 9am and 12pm to minimize the diurnal variations in sampling. The participants were instructed to rinse their mouth with water and wait for 10 minutes before commencing the saliva collection. Stimulated saliva was collected by asking the participants to chew on paraffin piece and then expectorating to pre-labeled disposable sterile plastic containers.The saliva produced in the first two minutes were discarded as it may cause analytical inaccuracy.
After the collection of the salivary samples, it was centrifuged at 8000g for 10 min. The supernatant thus obtained was used for the analysis of thiocyanate. The uric acid levels were analyzed using the uric acid estimation kit by AGAPPE Diagnostics. The pH of salivary samples were measured using the pH strips of BBR Chemocraft. The method of Lehti M et al., was used for the determination of salivary thiocyanate concentration [17]. Statistical Analysis was done and results were tabulated accordingly.
Results
The mean age of subjects in this study was 39.41 ±2.67 years. The frequency of tobacco consumption in each category is shown in [Table/Fig-1]. The corresponding CPI scores based on highest of all the six scores in an individual showed that non-tobacco users had highest percentage of healthy sextants and sextants with bleeding on probing. Proportion of participants with periodontal pockets of 4-5mm was highest among tobacco smokers (80.0%) and periodontal pockets of 6mm or more were observed to be higher among tobacco chewers (26.67%). The mean number of sextants with each CPI score are depicted below [Table/Fig-2].
When comparison of LOA scores were done between tobacco users and non users the result was statistically significant (p=0.033) [Table/Fig-3].
In the present study, when the salivary thiocyanate level were analysed it was found that the maximum level was detected in tobacco smokers (304 µg/ml) and tobacco chewers (290 µg/ml than non- tobacco users (132 µg/ml). The difference in salivary thiocyanate levels in saliva between the tobacco users and non- tobacco users was found to be highly statistically significant (p <0.001) [Table/Fig-4,5].
The salivary uric acid levels were analysed and it was found that tobacco users and non users had no significant difference [Table/Fig-6]. The Salivary pH assessment also showed that there is no significant difference seen between each category [Table/Fig-7].
When CPI scores were correlated with salivary thiocyanate level in tobacco smokers, it showed a positive correlation which was statistically significant (r= 0.506, p=0.004) . In tobacco chewers it showed a positive correlation (p= 0.331) but it was not statistically significant. Among non-tobacco users the CPI scores found to increase with the salivary thiocyanate level (r= 0.556) which was statistically significant (p = 0.001).
Discussion
Biomarkers of exposure are used to confirm the absorption of specific smoke constituents in a quantitative manner [18,19]. The present study showed significant differences in the periodontal status with respect to CPI scores and LOA scores in tobacco users and non- tobacco users. In tobacco users Deep periodontal pockets measuring 6mm or more were present whereas in non users there were no Deep pockets. These results are in agreement with the previous studies by Martinez Canut et al., Machuca et al., Alwahdni and Linden, Haber et al., Linden and Mullaly and Hashim et al., [20-25].
High prevalence of bleeding, calculus and periodontal pockets were observed among tobacco users compared to non- users. Loss of attachment was found to be more among tobacco smokers than non-users. In tobacco smokers the number of sites with probing depth and attachment loss could be explained on the ground that smoking diminishes both cell mediated and humoral immune responses. Oxygen uptake by polymorphonuclear cells (PMN) and production of oxygen radicals are severely compromised leading to dual impairments in chemotaxis and phagocytosis ability of leucocytes in smokers [26].
The periodontal destruction was seen high in tobacco chewers when compared to smokers and non-tobacco users. This may be due to the cumulative effect of placement of tobacco for longer duration in the mouth and also more irritants seen in smokeless tobacco products [27].
Increased prevalence and severity of periodontal destruction associated with smoking suggests that the host bacterial interactions normally seen in chronic periodontitis are altered resulting in more aggressive periodontal breakdown which may also be due to the release of tissue destructive enzymes. Hundreds of different compounds have been identified in tobacco smoke and some occur in concentrations judged to be harmful to health [28]. One of the study done by Germano et al., demonstrated the presence of complex glycocalyx structures, bacteriophage-like vesicles, spirochetes and bacterial co-aggregation by the Atomic force microscopy (AFM) analysis in periodontal diseases [29].
Our results demonstrated presence of higher amount of salivary thiocyanate level in tobacco users compared to non- tobacco users. Tobacco chewers had higher levels of thiocyanate in saliva compared to tobacco smokers. This may be due to longer duration of presence of tobacco in the oral cavity which possibly could increase the amount of production of thiocyanate levels in saliva. The level of thiocyanate levels were about twice the level in tobacco smokers than in non- tobacco users. This result is supported by another study done by Degiampietro F et al., [30].
Uric acid is the most important non- enzymatic antioxidant present in human saliva. The uric acid present in the saliva correlates with the plasma uric acid levels. Our present study showed that there is no much difference existed in the uric acid levels between tobacco users and non- tobacco users although it showed an association with the age (p=0.032). This is in agreement with the previous studies by Abdolsamadi HR et al., and Kondakova I et al., [13,14]. Uric acid is one of the most important antioxidants and contributes approximately 70% of the total salivary antioxidant capacity [31]. Tsuchiya et al., also demonstrated that smoking a single cigarette rapidly reduces the concentration of plasma antioxidants such as uric acid [32].
The salivary pH normally varies from 5.3 to 7.8. There are various sources of hydrogen ions in oral fluids; secretion by the salivary glands in the form of organic and inorganic acids, production by the oral microbiota, or acquisition through food. Our results showed no statistical difference between tobacco smokers, tobacco chewers and non- tobacco users. This findings were supported by other studies by Voelker MA et al., & Avsar A et al., [33,34]. Another study by Parvinen T et al., showed that the pH of saliva was lower in smokers compared to non- smokers [16].
Limitations
Several limitations of this study should be considered when interpreting the findings. First, the concentration of salivary uric acid was determined instead of the serum uric acid as there exist a difference between them. The concentration of salivary uric acid does not always directly correlate with the serum uric acid concentration.Secondly this study employed a cross sectional design, which might not have indicated temporal relationship between salivary thiocyanate, uric acid and pH and tobacco exposure. Thirdly subjects who have smoked 20 cigarettes or beedi for the last one week where selected. Therefore, information on light smokers or former smokers should be examined. The other limitation of this study was the age group. The particular age group (35-44 year) might also have age related periodontal destruction due to non tobacco usage related habits such as reduced fibroblastic activity and remodeling process, morphologic alterations, depression etc [35-37]. This age group was selected for study as this was the standard age group for monitoring the periodontal status of the adults [38].
The findings from this study concerning the fact that non-tobacco users exhibited a higher percentage of healthy periodontium compared to tobacco users and also a high concentration of salivary thiocyanate can be used as a biomarker of tobacco exposure. This study also indicates a relation between periodontal status and salivary thiocyanate levels in tobacco users, which opens the light to further studies in future. Nevertheless, the trends shown by this study do suggest possible avenues for future detailed investigation regarding the biological effects of tobacco usage.
Conclusion
Saliva (oral fluid) is a mirror of the body. Biomarkers in saliva like thiocyanate (SCN) can be a useful indicator of tobacco usage. In this study more periodontal destruction was seen in tobacco users than no tobacco users. Tobacco users had significantly higher concentration of SCN levels than non-users. The salivary uric acid levels were comparatively higher in tobacco users when compared to non- users, but it was statistically not significant. It was found that there was no statistical difference in Salivary pH between tobacco users and non-users. Informative biomarkers can further serve as early sentinels of disease, and this has been considered as the most promising alternative to classic environmental epidemiology.
[1]. B Rai, S Kharb, SC Kharb, Salivary enzymes and thiocyanate:salivary markers of periodontitis among smokers and non smokers.Advances in Medical and Dental Sciences. 2007 1:1-4. [Google Scholar]
[2]. MK Ah, GK Johnson, WB Kaldhal, KD Patil, KL Kalkwarf, The effect of smoking on the response to previous therapy.J Clin Periodontol 1994 21:91-97. [Google Scholar]
[3]. RS Field, JS Bravacos, CL Rose, Association between smoking different tobacco products and periodontal disease indexesJ Periodontol 1983 54:481-87. [Google Scholar]
[4]. BH Mullally, B Breen, GJ Linden, Smoking and patterns of bone loss in early onset periodontitis.J Periodontol 1999 70:394-401. [Google Scholar]
[5]. R Mohamed, JL Campbell, J Cooper-White, G Dimeski, C Punyadeera, The impact of saliva collection and processing methods on CRP, IgE, and Myoglobin immunoassays.Clin Transl Med 2012 1(1):19 [Google Scholar]
[6]. C Costagliola, Red cell reduced glutathione and tobacco smoke induced optic neuropathy.Metabolism, Pediatric and Systemic Opthalmology 1990 13:96-98. [Google Scholar]
[7]. U Cristenssen, UB Ericson, L Janzon, S Tibblin, A Melander, Influence of cigerette smoking on goiter formation, thyroglobulin and thyroid hormone levels in women.Journal of Clinical Endocrinology and Metabolism 1984 58:615-18. [Google Scholar]
[8]. H Fukayama, M Nasu, S Murakami, M Sugawara, Examination of antithyroid effects of smoking products in cultured thyroid follicles : only thiocyante is a potent antithyroid agent.Acta Endocrinologia 1992 127:520-25. [Google Scholar]
[9]. RB Hakim, JM Tielsch, Maternal cigarette smoking during pregnancy: a risk factor for childhood strabismusArchives of Ophthalmology 1992 110:1459-62. [Google Scholar]
[10]. LH Mosely, F Finseth, Cigarette smoking: impairment of digital blood flow and wound healing in the handHand. 1977 97(101) [Google Scholar]
[11]. PM Densen, B Davidow, HE Bass, EW Jones, A Chemical test for smoking exposure.Archives of Environmental Health 1967 14:865-74. [Google Scholar]
[12]. K Cherubini, CS Lorandi, SM Krapf, FR Sousa, LS Yurgel, MA Figueredo, FG Salud, Association between recurrent apthous stomatitis and Salivary Thiocyanate levels.J Oral Sci. 2006 48:153-56. [Google Scholar]
[13]. HR Abdolsamadi, MT Goodarzi, H Mortazavi, M Robati, F Ahmadi-Motemaye, Comparison of salivary antioxidants in healthy smoking and non-smoking men.Chang Gung Med J 2011 34(6):607-11. [Google Scholar]
[14]. I Kondakova, EA Lissi, M Pizarro, Total reactive antioxidant potential in human saliva of smokers and non-smokers.Biochem Mol Biol Int 1999 47:911-20. [Google Scholar]
[15]. G Maglis, H Verdugo, C Wiesner, E Cavajal, E Rossi, Determination of saliva pH in periodontal disease patients and a control group.Rev Dent Chile 1989 80:70-72. [Google Scholar]
[16]. T Parvinen, Stimulated salivary flow rate, pH and lactobacillus and yeast concentrations in non-smokers and smokersScand J Dent Res 1984 92:315-18. [Google Scholar]
[17]. M Lahti, J Vilpo, J Hovinen, Spectrophotometric determination of thiocyanate in human saliva.J Chem Ed 1999 76:1281-82. [Google Scholar]
[18]. SS Hecht, SG Carmella, M Chen, Quantitation of urinary metabolites of a tobacco-specific lung carcinogen after smoking cessationCancer Res 1999 59:590-96. [Google Scholar]
[19]. FJ Lowe, EO Gregg, M McEwan, Evaluation of biomarkers of exposure and potential harm in smokers, former smokers and never-smokersClin Chem Lab Med. 2009 47:311-20. [Google Scholar]
[20]. P Martinez-Canut, A Lorca, R Magán, Smoking and periodontal disease severity.J Clin Periodontol 1995 22:743-49. [Google Scholar]
[21]. G Machuca, I Rosales, JR Lacalle, C Machuca, P Bullón, Effect of cigarette smoking on periodontal status of healthy young adults.J Periodontol 2000 71:73-78. [Google Scholar]
[22]. A Al-Wahdni, GJ Linden, The effects of cigarette smoking on the periodontal condition of young adults.J Clin Periodontol 2003 30:132-37. [Google Scholar]
[23]. J Haber, J Wattles, M Crowley, R Mandell, K Joshipura, RL Kent, Evidence for cigarette smoking as a major risk factor for periodontitis.J Periodontol 1993 64:16-23. [Google Scholar]
[24]. GJ Linden, BH Mullally, Cigarette smoking and periodontal destruction in young adultsJ Periodontol 1994 65:718-23. [Google Scholar]
[25]. R Hashim, WM Thomson, ARC Pack, Smoking in adolescence as a predictor of early loss of periodontal attachment.Community Dent Oral Epidemiol 2001 29:130-35. [Google Scholar]
[26]. MJ Pabst, KM Pabst, JA Collier, Inhibition of neutrophil and monocyte defensive functions by nicotineJ Periodontol 1995 66:1047-55. [Google Scholar]
[27]. MP Walsh, BJ Ebstein, The oral effects of smokeless tobaccoJ Can Dent Assoc 2000 66:5-22. [Google Scholar]
[28]. RM Palmer, RF Wilson, AS Hasan, DA Scott, Mechanisms of action of environmental factors – tobacco smoking.J Clin Perio 2005 32:180-95. [Google Scholar]
[29]. F Germano, E Bramanti, C Arcuri, F Cecchetti, M Cicciù, Atomic force microscopy of bacteria from periodontal subgingival biofilm: Preliminary study resultsEur J Dent 2013 7:152-58. [Google Scholar]
[30]. P Degiampietro, E Peheim, D Drew, H Graf, JP Colombo, Determination of thiocyanate in plasma and saliva without deproteinization and its validation as a smoking parameter.J Clin Chem Clin Biochem 1987 25:711-17. [Google Scholar]
[31]. RM Nagler, I Klein, N Zarzhevsky, N Drigues, A Rezinck, Characterization of the differentiated antioxidant profile of human saliva.Free Radic Biol Med 2002 32:268-77. [Google Scholar]
[32]. M Tsuchiya, A Asada, E Kasahara, EF Sato, M Shindo, M Inoue, Smoking a single cigarette rapidly reduces combined concentrations of nitrate and nitrite and concentrations of antioxidants in plasmaCirculation. 2002 105:1155-57. [Google Scholar]
[33]. MA Voelker, M Simmer-Beck, M Cole, E Keeven, D Tira, Preliminary Findings on the Correlation of Saliva pH, Buffering Capacity, Flow, Consistency and Streptococcus mutans in Relation to Cigarette SmokingJ Dent Hyg 2013 87(3):118-33. [Google Scholar]
[34]. A Avsar, O Darka, B Topaloglu, Y Bek, Association of passive smoking with caries and related salivary biomarkers in young childrenArch Oral Biol 2008 53:969-74. [Google Scholar]
[35]. E Krieger, S Hornikel, H Wehrbein, Age-related changes of fibroblast density in the human periodontal ligamentHead Face Med 2013 :9-22. [Google Scholar]
[36]. SGF Gomes, CB Meloto, W Custodio, CM Rizzatti-Barbosa, Aging and the periodontiumBraz J Oral Sci 2010 9(1):1-6. [Google Scholar]
[37]. CF Andreescu, LL Mihai, M Raescu, MJ Tuculinã, C Cumpãtã, DL Ghergic, Age influence on periodontal tissues: A histological studyRom J Morphol Embryol 2013 54:811-15. [Google Scholar]
[38]. Oral Health Surveys-Basic Methods 2013 5th EditionGenenvaWHO:9-22. [Google Scholar]