Introduction
Langerhans cells (LCs), are dendritic cells of the epithelium which play a role in an array of oral lesions from gingivitis to oral cancer. Oral Submucous Fibrosis (OSMF), a potentially malignant disorder (PMD), is an insidious chronic disease with juxta-epithelial inflammatory changes leading to fibrosis. Langerhans cells (LCs) may play a part in the ongoing inflammatory dysregulation of OSMF.
Objective
The study was aimed at elucidating the distribution of LCs in varying grades of OSMF.
Materials and Methods
A retrospective study using 39 cases of OSMF, graded using Haematoxylin and Eosin (H&E) stained section. Immunohistochemistry was performed using polyclonal anti- CD1a antibodies to identify LCs in 5 cases of normal tissue and 39 samples of OSMF. The distribution of LCs among the various grades and normal mucosa analysed using Mann-Whitney U test.
Results
LC population in the OSMF was significantly higher when compared to the normal epithelium (p<0.001). Within the grades the advanced stage had more LCs than the other stages.
Conclusion
The increase in LCs might indicate the role of antigenic exposure in turn leading to cell mediated immunity in OSMF. Thus the fibrosis in OSMF might have a direct link to LCs.
Introduction
Langerhans cells (LCs) dendritic, non-keratinocytic clear cells of the oral epithelium present in the suprabasal layer are derived from myeloid stem cells of bone marrow. They are Antigen Presenting Cells (APCs) that aid to provoke a specific T cell reaction by the interaction of the MHC class II with the CD4+ cells. In the oral cavity, they are associated with the immunopathogenesis of various lesions such as gingivitis and periodontitis, oral lichen planus, contact hypersentivity, recurrent aphthous Stomatitis and a plausible role in oral cancer has also been demonstrated [1]. They belong to the family of dendritic cell system (DCS) among which LCs can be differentiated from others by the presence of Birbeck granules [2]. Oral Submucous Fibrosis (OSMF), a potentially malignant disorder is invariably associated with an inflammatory process which causes the release of fibrogenic cytokines such as TGF-β, IL-6, TNF and IF-α. This is primarily due to the presence of activated T lymphocytes [3]. Haque et al., provided direct evidence for an ongoing cell mediated immunity in OSMF [4]. Increased dendritic cells also noted in the epithelium of OSMF patients. Chiang et al., further established the role of cell mediated immunity in the pathogenesis of OSMF by demonstrating an increased number of T cells over B cells and also found that CD4+ cells were significantly higher than the CD8+ cells [5]. The increased dendritic cells and cytokines suggest that LCs might recognise the unknown antigen in OSMF which migrates with the support of chemokines expression through the lymphatics and promote CMI. Therefore, we aimed at evaluating the distribution of LCs in various stages of histopathologically diagnosed oral submucous fibrosis as LCs are involved in stimulating T cell reaction. T cells in turn release various cytokines such as IL-6, TNF and IF-α and growth factors like PDGF and TGF-β [6,7] leading to fibrosis [8].
Materials and Methods
Thirty nine patients previously clinically and histopathologically diagnosed as OSMF and five patients with normal mucosa diagnosed histopathologically were retrieved randomly from the archives of Department of Oral Pathology, after obtaining ethical clearance from Sri Ramachandra University. The control samples were collected from apparently normal buccal mucosa and histopathologically confirmed for the absence for any immunological reaction. The OSMF cases were graded according to Pindborg JJ and Sirsat SM staging [9] as, very early stage, early stage, moderately advanced stage and advanced stage using Haematoxylin and eosin stained sections. Very early and early stages were grouped together as mild stage. Skin epidermis was selected as the immunohistochemistry protocol control.
Immunohistochemistry: Paraffin embedded specimens were sectioned of 4μm thickness on charged slides (Star Frost, Leica Ltd), placed in warmer at 60°c for 5 minutes, followed by deparaffinization in two changes of xylene for five minutes each. After which three changes of alcohol each for five minutes was performed. Heat induced antigen retrieval done. The staining procedure was according to manufacturer’s protocol as described below. Tris buffer wash was performed thrice for five minutes each; it was followed by peroxide block for five minutes. After Tris buffer washes, super block (Scytek Laboratories, U.S.A) was applied for fifteen minutes which was followed by incubation of Primary antibody, polyclonal anti-CD1a antibody (Quartett, Germany) for thirty minutes. Anti- Polyvalent HRP polymer(secondary antibody) (Scytek Laboratories, U.S.A) incubated for one hour followed by three Tris buffer washes for five minutes each followed for incubation with DAB substrate and DAB mixture for one minute. It was counter stained by Harris Haematoxylin. Normal human skin was used as control (positive and negative) tissue.
Evaluation: The stained slides were viewed by two investigators and analysed under light microscope to eliminate inter-observer bias. Using a five header microscope the disagreements were resolved. Brown surface stained cell was taken as positive for CD1a as CD1a is a surface marker. The number of LCs per high power field (400x) were counted from 6 fields in the varying layers of epithelium and the average LCs in a high power field was calculated for each of the sections. The presence of LCs if present in the connective tissue was noted.
Statistical Analysis
Statistical analysis was performed using Mann-Whitney U analysis to determine the difference in the number of LCs between normal epithelium and OSMF tissues. ANNOVA and Mann-Whitney U-test employed for comparison between various grades and between mild and moderately advanced group with the advanced stage respectively.
Results
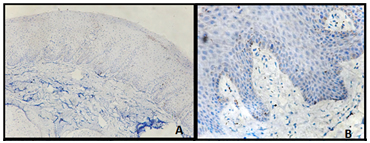
The OSMF sections were graded with H&E sections, by two observers to overcome inter-observer variation, out of which 21 were mild, 11 were moderately advanced and 7 advanced stage of OSMF. The protocol control–skin epidermis showed positivity in the positive control and the negative control (devoid of primary antibody) was completely negative. In the normal epithelium (study control), the LCs stained by CD1a antibody were found to be restricted to the suprabasal layer with an average cell count of 3 per high power field [Table/Fig-1a,b].
In OSMF tissues there was a considerable increase in LCs with an average cell count of 12 cells per high power field in the 39 cases. There was a significant difference in the number of LCs between normal and OSMF tissues (p value < 0.001) [Table/Fig-2].
The cells were distributed suprabasally, in spinous layer and few in the superficial layers. Morphological alterations were the presence of a prominent dendritic morphology of the cells appeared in OSMF tissues when compared to normal tissues. The cell count distribution in the various stages of OSMF is tabulated [Table/Fig-3].
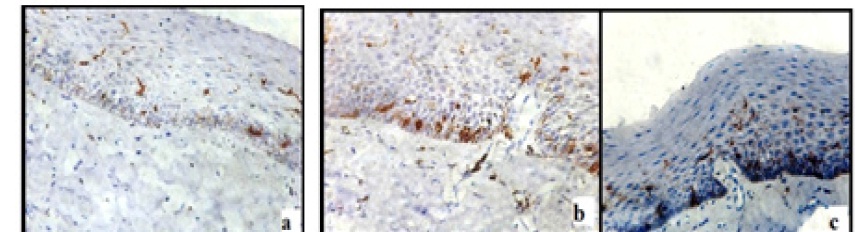
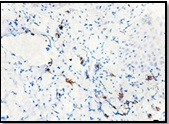
Few moderately advanced and most advanced stage of OSMF showed highest number of LCs [Table/Fig-4a-c]. In the moderately advanced cases the CD1a positive cells were found in the connective tissue which showed characteristic dendritic morphology. These cells were predominantly present in the juxta-epithelium [Table/Fig-5].
ANOVA test comparing the LC distribution between the three stages was not significant (p-value=0.10). When the LCs expression was compared between two groups, mild and moderately advanced grade with advanced grade was significant with p-value < 0.001 using Mann-Whitney U-test.
Discussion
OSMF has an unclear aetiology. A strong association with betel quid chewing demonstrated which causes a favourable environment by chemical (arecoline) and irritant (microtrauma) leading to fibroelastic changes. OSMF bears an ongoing immunological process which leads to altered release of various cytokines effectively causing dysregulation in the collagen formation and degradation. In this study we have aimed to establish the role of antigen presenting cells which plays a pivotal role in initiating and modulating immunity.
Haque et al., [4], demonstrated the predominance of CD3 and CD4 positive cells. A striking finding was the presence of HLA-DR dendritic cells in the epithelium and lamina. These dendrites appeared to face the epithelium simulating a picture of an underlying antigenic response. Our study showed increase in the Langerhans cells in the OSMF epithelium when compared to normal epithelium with active dendritic morphology in OSMF tissues which further emphasizes the role of T cell immunity in OSMF.
Chiang et al., [5], demonstrated increase in T cell density in moderately advanced cases than the advanced cases which was comparable to the normal tissue in the connective tissue. In our study there was presence of interstitial LCs in the connective tissue indicating an ongoing immune response in the moderately advanced cases and a role of an unknown antigen eliciting the response that can aid to explain the increase in density of T cells in this stage.
On the contrary, their study showed similar density of T cells in both the stages in the epithelium. Our study showed an increase in LCs in the advanced stage than the other two which might be attributed to the continuous antigenic exposure. Though Langerhans cells release IL-1α leading to increase in collagenase [10], the simultaneous activation of T cells topples the effect by the release of fibrogenic cytokines.
Haque et al., verified that OSMF tissues showed high localization of IL-6, Fibroblast growth factor (FGF), platelet derived growth factor(PDGF) and IL-1. An altered cytokine profile in peripheral blood of OSMF patients where an increased IL-6, IL-8, IL-1β and TNF-α than in normal individuals was also demonstrated [4,7] thereby suggesting their role in altered collagen metabolism.
Nissen et al., demonstrated an abnormal increase of LCs in the hypertrophic scars, and hypertrophic scars remaining hypertrophic even after 12 months of follow up suggesting their role in scar tissue formation by altered matrix production [11]. In a similar way, an aberrant epithelial regulation indicated by the marked raise in the LCs in OSMF as seen in our study, either through the keratinocytes or directly on the fibroblasts, has a pivotal role in fibrosis.
Yong Xie et al., demonstrated increased active LCs expression in cases of localised scleroderma suggesting that LCs might play a paramount role in this autoimmune disorder [12]. An autoimmune aetiology has also been suggested in OSMF as autoantibodies in the sera of patients have been reported [3].
On analysing the results of the study, it can be concluded that LCs may play a role in the fibrosis in OSMF directly or indirectly. The limitations of the study include lack of information on the duration and constituents of the deleterious habits and usage of a purely histopathological staging for the analysis.
Role of LCs have been discussed in state of dysplasia and oral squamous cell carcinoma (OSCC) where there is increase in LCs expression in the submucosa whereas negative correlation has been noted in the higher grades of OSCC. This illustrates that LCs play a role in presenting the altered antigens in dysplastic cells and the decrease in the immune status in OSCC patients in turn diminishes the Langerhans activity [13-15]. Due to the malignant potential of OSMF, observation of changes of LCs in these lesions may throw an insight in the malignant transformation but there is no literature evidence to directly suggest the LCs expression in OSCC transformed from an OSMF.
CD1a expression in normal tissues confined to the parabasal layers (a&b)
Expression of CD1a in normal and OSMF tissues
| No. Of samples | Average LCs per field ±S.D | Mean Rank | S.E mean |
|---|
| Normal | 5 | 2.73±.45 | 4.20 | .20 |
| OSMF | 39 | 11.53±6.47 | 24.85 | 1.04 |
Distribution of Langerhans cells (LCs) in varying grades of Oral submucous Fibrosis (OSMF)
| Grade | No. of Samples | Average cells per field Mean±S.D |
|---|
| Mild | 21 | 10.18±6.00 |
| Moderately Advanced | 11 | 11.14±5.18 |
| Advanced | 7 | 16.21±8.25 |
Presence of LCs with prominent dendritic processes distributed among various layers of epithelium in moderately advanced OSMF (a) and advanced OSMF (b&c)
Moderately advanced OSMF showing active dendritic cells seen in juxtaepithelial connective tissue stroma
Conclusion
Our study shows a definitive increase in LCs expression in the OSMF which can either suggest a role langerhans cells to alter the epithelial characteristics and alter the submucosal matrix deposition or act as only as APC cells to unknown antigens or have any direct influence on the fibroblasts, the mechanism still requires clarification.
[1]. S Jaitley, TR Saraswathi, Pathophysiology of Langerhans cellsJ Oral Maxillofac Pathol 2012 16:239-44. [Google Scholar]
[2]. T Lombardi, C Hauser, E Budtz Jorgensen, Langerhans cell: structure, function and role in oral pathological conditionsJ Oral pathol med 1993 22:193-202. [Google Scholar]
[3]. WM Tilakaratne, MF Klinikowski, T Saku, TJ Peters, S Warnakulasuriya, Oral submucous fibrosis: Review on aaetiology and pathogenesisOral Oncology 2006 42:561-68. [Google Scholar]
[4]. MF Haque, M Harris, S Meghji, PM Speight, An immunohistochemical study of oral submucous fibrosisJ Oral Pathol Med 1997 26:75-82. [Google Scholar]
[5]. CP Chiang, HY Wu, BY Liu, JT Wang, MYP Kuo, Quantitative analysis of immunocompetent cells in oral submucous fibrosis in TaiwanOral Oncology 2002 38:56-63. [Google Scholar]
[6]. J Banchereau, RM Steinman, Dendritic cells and the control of immunityNATURE 1998 392:245-52. [Google Scholar]
[7]. MF Haque, M Harris, S Meghji, AW Barrett, Immunolocalization of cytokines and growth factors in oral submucous fibrosisCytokine 1998 10:713-19. [Google Scholar]
[8]. MF Haque, S Meghji, U Khitab, M Harris, Oral Submucous fibrosis patients have altered levels of cytokine productionJ Oral Pathol Med 2000 29:123-28. [Google Scholar]
[9]. K Ranganathan, G Mishra, An overview of classification schemes for oral submucous fibrosisJ Oral Maxillofac Pathol 2006 10(2):55-59. [Google Scholar]
[10]. H Matsue, PDJ Cruz, PR Bergstresser, A Takashima, Cytokine expression by epidermal cell subpopulationsJ Invest Dermatol 1992 99:42S-45S. [Google Scholar]
[11]. FB Niessen, J Schalkwijk, H Vos, W Timens, Hypertrophic scar formation is associated with an increased number of epidermal Langerhans cellsJ Pathol 2004 202:121-29. [Google Scholar]
[12]. Y Xie, X Zhang, Y Inoue, S Wakasugi, T Makino, H IHN, Expression of CD1a and CD86 on scleroderma Langerhans cellsEur J Dermatol 2008 18(1):50-54. [Google Scholar]
[13]. RB Upadhyay, J Upadhyay, NN Rao, P Agrawal, The role of Langerhans cells in oral squamous cell carcinomaChinese-German Journal of Clinical Oncology 2011 10(10):606-61. [Google Scholar]
[14]. J Ohman, B Magnusson, E Telemo, M Jontell, B Hasseusn, Langerhans Cells and T Cells Sense Cell Dysplasia in Oral Leukoplakias and Oral Squamous Cell Carcinomas – Evidence for ImmunosurveillancScandinavian Journal of Immunology 2012 76:39-48. [Google Scholar]
[15]. TJ Lasisi, AO Oluwasola, OA Lasisi, EE Akang, Association between langerhans cells population and histological grade of oral squamous cell carcinomaJ Oral Maxillofac Pathol 2013 17:329-33. [Google Scholar]