Introduction
Periodontitis, a destructive chronic inflammatory disease, results from a polymicrobial infection and is characterized by the destruction of tooth supporting tissues including the alveolar bone. There is now a consensus that Campylobacter rectus (C.rectus) is one of the bacteria strongly associated with periodontitis. C.rectus, a gram-negative, microaerophilic, and motile bacterium, has been thought to play a pathogenic role in humans. Campylobacter species are commonly found in the subgingival sites. Elevated levels of Campylobacter species have been reported in human periodontal disease [1-5]. Extraoral infections by these organisms, however, are rarely reported [6]. Levels of Campylobacter species such as rectus and gracilis were elevated in diseased human subgingival sites compared with non-diseased sites, suggesting direct association with periodontal disease [7].
Molecular methods, such as the polymerase chain reaction (PCR) method, overcomes many of the problems associated with traditional phenotype-based identification methods. It is widely used to identify microbial species that are difficult to cultivate, uncultivable species, and specific-species strains that show a phenotypically divergent behaviour and thereby are difficult to be identified by culture procedures. Under optimized conditions 16s rDNA PCR methodology shows high specificity and has the highest detection rate among the microbiological identification methods [7].
Most micro-organisms require iron for its growth and survival. The ability of pathogens to obtain it from its host is one of the critical determining factors. In humans, levels of free ionic iron are available in low amount [8,9]. Human gingival crevicular fluid also contains iron-containing proteins such as transferrin, haemoglobin and lactoferrin, through which bacteria acquires iron [10]. Transferrin, a serum glycoprotein possessing two iron-binding sites, is important for rendering iron unavailable to bacteria in-vivo [8]. The presence of transferrin was correlated with severity of disease [11]. Hence, its level in subgingivL sites may represent an important source of iron for periodontopathogens in periodontitis. Numerous periodontopathogens require iron constituents for their growth and metabolism in subgingival crevice [11,12].
It is known that C.rectus was almost detected in increasing pocket depth [13,14]. Few studies reported the ability of C.rectus to utilize iron for its growth. Recently it was demonstrated that C.rectus isolated from periodontitis sites has the capacity to imbibe iron from transferrin in a ferric reductive dependent pathway in-vitro to support its growth [10]. However, in-vivo studies are lacking. It is not known whether C.rectus has any effect in serum levels of iron binding and carrying capacity. Therefore, in the present study we evaluated effects of quantification of C.rectus on serum iron, total binding capacity and transferrin levels in chronic periodontitis and healthy sites. C.rectus was identified and quantified by 16S rDNA based PCR analysis.
Materials and Methods
This cross-sectional study was carried out within a span of one year in the age group of ≥18 years in both males and females. Total of 120 subjects were included and divided into test and control groups. The test group included 60 subjects with chronic generalized periodontitis and control group comprised of 60 subjects who were otherwise healthy. All subjects were recruited randomly from the outpatient, Department of Periodontology, Sinhgad Dental College and Hospital, Pune after considering inclusion criteria and after obtaining institutional ethical clearance.
Chronic generalized periodontitis [15] subjects who had probing pocket depth and periodontal attachment loss (PAL) of ≥ 5mm and presence of gingival bleeding in ≥ 10% sites were included in test group. Subjects with probing pocket depth and PAL of ≤ 3mm and ≤ 10% sites exhibiting bleeding on probing were included in control group. Any history of smoking, systemic diseases, administration of antibiotics, anti-inflammatory or any other drugs for atleast six months, history of periodontal therapy in last 12 months and pregnant and lactating females were excluded from both study groups.
Subjects were informed of the purpose and protocol of the study and consent was obtained. All subjects were enrolled for haematological and microbiological examination. Probing depth and PAL were measured with the help of a Williams graduated periodontal probe.
Collection of plaque sample: Ultrasonic unit was used for removal of supragingival plaque and calculus of the target region. Under proper isolation with cotton rolls and suction unit, sterile gracey curette was used to collect plaque sample from three deepest subgingival sites of PAL of ≥ 5mm per subject of chronic periodontitis and from three sites of PAL ≤ 3mm in healthy subjects. It was then immediately placed in eppendorf tube containing TE buffer. The tube was sealed, marked with patients name and age, and transported to microbiological laboratory within 24 hours.
DNA Extraction Procedure: Within 48 hours, DNA was extracted according to Modified Proteinase-K method [16]. The tube was centrifuged at 5,000 rpm for 5 min. The supernatant that was formed was discarded. Fresh TE buffer (500 μl) was added and centrifuged for 3-4 minutes. The above procedure was repeated for 3-4 times with fresh TE buffer. Supernatant was discarded and 50 µl lysis buffer I was added, vortexed and kept for 5 mins.,then 50 µl lysis buffer II and 10 µl proteinase – K (100 µg/ml) was added and vortexed vigorously. It was kept in water bath for 2 hours in 60°C and then in boiling water bath for 10 minutes. The resultant sample that was obtained was DNA which was stored at -200C. The purity of RNA was checked by electrophoresis.
PCR Amplification: The PCR Master mix (Chromous Biotech Pvt. Ltd, Bangalore, India) (Taq polymerase buffer containing 1.5 mM MgCl2 – 10X, dNTP mix - 10 mM, and Taq polymerase enzyme - 1.5U/reaction) was used. Twenty five µl of above master mix was taken to thermostable PCR tubes, 1.2 µl each of Forward (5’ TTT CGG AGC GTA AAC TCC TTT TC 3’) and Reverse (5’ TTT CTG CAA GCA GAC ACT CTT 3’) oligonucleotide primers (Bioserve Biotech India Pvt. Ltd, Hyderabad, India) specific for C.rectus was added (30 pmole). Five µl template DNA (<00 ng) was added and finally the total volume was made to 50 µl by adding water. A premixture was prepared and aliquoted into each tube. The 50 µl/aliquot of premix Qiagen Taq core kit (Qiagen India Pvt Ltd, New Delhi, India) containing 3 U/μL Taq polymerase, 2xCoral load buffer, 4 mM Mgcl2, 0.4 mM of each dNTP was added.
After thawing, the PCR master mix was gently vortexed and, briefly centrifuged. A thin walled PCR tube on ice and both 1 µl (25 pmole)forward and reverse primer mixes, 5 µl (10 pg - 1 μg) template RNA, 2 μl dNTP mix, 0.5 µl Taq polymerase enzyme, 5 µl coral load buffer and water added to make final volume of 50 µl reaction. The samples were gently vortexed and spun down. The tubes were kept in a thermal cycler (Applied Biosytem, California, USA) for carrying out initial denaturation (950C, 5min) followed by denaturation (950C, 1min), annealing (540C, 30 sec) and extension (720C, 1min) repeated for 35 cycles before final extension (720 C for 5 min) to allow all extensions to be completed for amplification. Samples were kept at 40o C following PCR method.
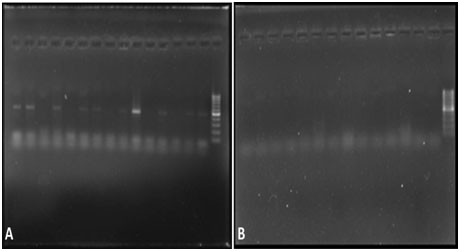
Detection of Amplified Products [17]: Amplified products were subjected to gel electrophoresis (Biobee Tech, Bangalore, India) through 1.5% agarose gel containing 1x TAE, 20 µl of each amplified product mixed with 3 µl of bromophenol blue loading dye. Electrophoresis was performed at 25V for 2 hour. The gel was visualized under UV light illuminator gel (Major Science, California, USA) after staining with ethidium bromide (0.5 μg/ml). The size(s) of PCR products were determined by comparison with a DNA ladder (100 bp), run simultaneously on the alongside of PCR products. The markers were seen on monitor screen. The detected organism was quantified and analyzed by Total lab Quant software (Total Lab software, Newcastle upon Tyne, UK).
Collection of Blood Sample Under aseptic precautions and tourniquet application, a 22 gauge needle was used to withdraw around 5 ml of venous blood in non-fasting state and collected in vacutainers. Blood was then centrifuged in REMI R-8C laboratory centrifuge machine and plasma separated and sent to the laboratory for the estimation of serum iron, total iron-binding capacity (TIBC) and transferrin levels.
Serum Iron and Unsaturated Iron-Binding Capacity [18]: The modification of the Persijn method was used. Test readings were recorded with zero spectrophotometer at 560 nm against reagent blank before (A1) and after (A2) addition of Iron color reagent. The expected serum iron levels of 60 - 150 µg/dl were considered as normal.
TIBC [18]: It is the sum of serum iron and unsaturated Iron-binding capacity. The expected serum iron levels of 250 - 400 µg/dl was considered as normal.
Transferrin (Immunoturbidimetric test, 5 + 1) [19,20]: It is a photometric measurement of the antigen-antibody reaction between goat-Antibody on human transferrin and TRF in the sample. The concentration in unknown samples is calculated through a calibration curve using a suitable mathematical procedure e.g. logit/log. The calibrations curve is established by 5 calibrators of different concentrations and NaCl-solution (9 g/l) for zero. The sensitivity or lower detection limit is 3 mg/dl. The expected normal range of serum transferrin is 200 – 360 mg/dl (2.0 – 3.6 g/l)
Statistical Analysis
Data was compiled and SPSS software 17 (SPSS Inc. Chicago, USA) was used for statistical analysis. Mann-Whitney test was used for quantitative assessment of bacteria in both groups. Chi-Square test was used to compare serum levels of iron, TIBC and transferrin in both groups. The effect of quantification of C.rectus on serum components was assessed by regression analysis (curve fit estimation). The p-value of 5% was considered to be statistically significant.
Results
Total 120 subjects were included after considering the inclusion and exclusion criteria of the groups. Out of them 74 were male and 46 were female subjects. The total frequency and prevalence of occurrence of C. rectus in both groups was 26 in males (n=42) and 16 in females (n=42). In present study, 110 subjects agreed for blood investigations. Six subjects’ in chronic generalized periodontitis and 4 in control groups didn’t agree for blood examination.
Total of 24 chronic periodontitis and 18 healthy subjects showed occurrence of C. rectus which was statistically non-significant (p > 0.05) [Table/Fig-1]. The comparison of quantification of C.rectus in both groups showed statistically significant difference (p <0.001) [Table/Fig-2,3a,b]. In chronic periodontitis, iron levels were within normal limits, TIBC and transferrin levels were raised in 12 subjects. In healthy group, 6 subjects showed elevated serum iron levels, TIBC and transferrin elevated in 6 and 8 subjects respectively [Table/Fig-4]. Serum iron levels were significant in healthy group. However, comparison of raised levels of serum TIBC and transferrin in both study groups showed non-significant differences (p = 0.103; p = 0.281 respectively).
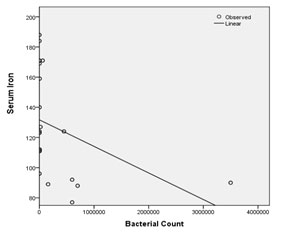
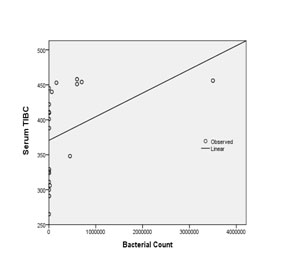
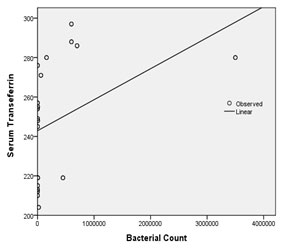
Regression analysis was performed to find out the effect of quantified bacterial counts on host blood serum parameters in both groups. TIBC and transferrin levels were constantly elevated, while serum iron showed negative correlation with increasing bacterial counts. Significant results were obtained upon comparison (serum iron, p = 0.010; TIBC, p = 0.010; transferrin, p = 0.009 respectively) [Table/Fig-5,6,7].
Discussion
C.rectus has a potential role in chronic periodontitis. Studies have reported higher prevalence of C.rectus in periodontitis sites compared to healthy [3-5]. Few studies reported significant differences in healthy and periodontitis sites [7,21,22]. Ihara H et al., found C.rectus correlated significantly with clinical findings such as probing depth, bleeding on probing and gingival index [14]. Lower prevalence was also detected in some studies in diseased sites [17,23]. Our study showed significant increase in bacterial count in severe periodontitis sites. This finding was similar to Macuchi et al., [6] and Grenier D et al., [10]. This suggests that C.rectus is an amphibiotic opportunistic Microorganism, not an exogenous pathogen localized in deep pockets.
There is convincing literature of importance of iron in host-microbial interaction. The ability to obtain iron from their host is a critical factor for bacterial survival. In humans it is available in abundance in extracellular fluids, but the amount of free ionic iron (10-18 M) is far too low to support growth of most bacteria [8,9]. Its concentration in GCF is reported to be 20-40 µM (70 % of serum) [24]. In tissues, it is mainly transported, absorbed by serum glycoprotein Transferrin. Goulet et al., reported the effect of gingipains on transferrin and their fragments in GCF from periodontitis sites and correlated with the severity of the disease [11]. The transferrin concentrations in GCF samples were significantly higher in diseased sites than healthy periodontal sites [25].
Different microorganisms adapt several mechanisms to acquire iron as their nutrient source from their host. Porphyromonas gingivalis, Prevotella nigrescens and Prevotella intermedia binds to transferrin through their cell surface receptors [26,27]. Gingipains causes proteolytic cleavage of transferrin, release and uptake of iron [11].
This study evaluated the ability of C.rectus to utilize human transferrin as a source of iron. In the clinical laboratory, transferrin is determined mainly for the investigation of iron metabolism. The determination of TIBC therefore is a physiological approach. Serum iron samples were significantly raised in healthy group compared to study group. In contrary, there were no significant elevation in serum TIBC and transferrin levels in both groups. Mukerjee reported three-fold increase in iron level in crevicular fluid in ligature-induced perioodontitis [28]. In our study, although serum levels were not raised in chronic periodontitis subjects; most of them had a higher range compared to healthy subjects.
This study evaluated whether the increased counts of C.rectus bear any effect on serum iron components. The regression analysis showed higher bacterial quantification, which was constantly associated with decrease in iron levels and increase in TIBC and transferrin levels in-vivo. This difference was statistically significant. Grenier and Tanabe found C.rectus cells were able to efficiently remove and assimilate iron bound to transferrin. The iron-uptake was found to be significantly increased when C. rectus cells were grown under iron-restricted conditions [15]. Our study findings were in accordance with these findings. The in-vivo findings suggest C.rectus requires iron as a significant source of nutrition for its survival and growth from its hosts in deeper subgingival sites. Similarly, other gram-negative periodontopathogens use transferrin as their nutrient source for their establishment.
Some limitations should be considered while interpretation of preliminary findings of this study. In this cross-sectional study, few subjects in the study and control groups refused to issue blood samples. Variations in bacterial strain, geographical distribution of C.rectus are other limiting factors.
Deep subgingival sulcus area serves as a niche for C.rectus survival in addition to other areas such as tongue, cheek mucosa, and saliva [29,30]. Studies have reported that poor oral hygiene was associated with fatal infections viz. chest wall [31], breast wall [32], vertebral abscess [33], gastroesophageal carcinoma [34], from infected skin and a soft tissue bite wound [35], necrotizing soft tissue infection of right thigh, right empyema thoracis and ruptured mycotic intracranial aneurysm with subdural empyema [36]. In-vivo animal study showed C.rectus has the competence to translocate by haematogenous route to the fetoplacental unit and placental inflammation during early gestation [37,38] Further, higher levels of C.rectus were correlated with salivary estradiol concentrations and sites with pocket depth in pregnant females [39]. Camphylobacter species are commonly known to cause gastrointestinal diseases in humans. C.rectus, harbored in periodontitis-associated oral biofilms, appeared to be an important predisposing factor for invasive systemic infections in susceptible individuals. These bacteria may also contribute the recurrence or reinfection. Periodontal therapy results in elimination or reduction in population of C.rectus and other putative pathogens. Consequently, the chances of developing or acquiring infections elsewhere in other parts of human body can be reduced significantly. The effects of oral C. rectus infection on systemic health remain to be determined.
Long-standing chronic periodontal inflammation show signs of anemia. Haemoglobin, number of erythrocytes and haematocrit levels were in lower chronic periodontitis [40,41]. Deficiency of iron and increased demand of iron-binding components were noted in conditions like iron-deficiency anemia. In such conditions, whether C.rectus count remains unaltered, or significantly reduced in count needs to be assessed. Further studies are required to find out the significance of C.rectus in subgingival pockets and its effects on the systemic health of an individual or vice-versa.
Occurrence of C. rectus in chronic periodontitis and healthy groups
| PCR | Chronic Periodontitis | Healthy | Total | p-values |
|---|
| Absent | 36 | 42 | 78 | 0.417 |
| Present | 24 | 18 | 42 |
| Total | 60 | 60 | 120 | |
Bacterial quantification of C. rectus in chronic periodontitis and healthy groups significant
| Category | N | Mean Rank | Sum of Ranks | p-value |
|---|
| Chronic Periodontitis | 24 | 29.42 | 706.00 | < 0.001* |
| Healthy | 18 | 10.94 | 197.00 |
Detection of C.rectus in chronic periodontitis (a) and healthy group (b)
Comparison of serum levels with chronic periodontitis and healthy groups significant
| Serum Levels | Chronic Periodontitis | Healthy | Total | p-value |
|---|
| Iron | Raised | 0 | 6 | 6 | 0.013* |
| Normal | 54 | 50 | 104 |
| TIBC | Raised | 12 | 6 | 18 | 0.103 |
| Normal | 42 | 50 | 92 |
| Transferrin | Raised | 12 | 8 | 20 | 0.281 |
| Normal | 42 | 48 | 90 |
Comparison of serum iron with bacterial quantification by regression analysis
Comparison of serum TIBC with bacterial quantification by regression analysis
Comparison of serum transferrin with bacterial quantification by regression analysis
Conclusion
In summary, preliminary findings provide valuable information on C.rectus. The higher quantification of C.rectus was observed in chronic periodontitis. This higher count was significantly associated with elevated serum levels of TIBC and transferrin and decreased iron levels in vivo. Further longitudinal studies on larger population are warranted. Different mechanisms by which bacteria bind to transferrin and its interactions at the extracellular and cellular levels and iron acquisition by C.rectus in-vivo are necessary to confirm the findings.
Acknowledgements
I wish to express my heartfelt thanks and sincere gratitude to Dr.Shobha More, Dr. Kishore Bhatt and Dr. Vinayak Joshi for their valuable guidance and for conducting the PCR test. I would also like to thank Dr. Dheeraj Kalra for his swift assistance in statistical analysis.
[1]. MJ LaGier, DS Threadgill, Identification of novel genes in the oral pathogen Campylobacter rectusOral Microbiol Immunol 2008 23:406-12. [Google Scholar]
[2]. A Tanner, MFJ Maiden, PJ Macuch, LL Murray, JR Kent, Microbiota of health, gingivitis, and initial periodontilisJ Clin Periodontol 1998 25:85-98. [Google Scholar]
[3]. RA Habashneh, JA Karasneh, YS Khader, Predominant microflora in chronic and generalized aggressive periodontitis in a Jordanian population.Dentistry. 2014 4:1-9. [Google Scholar]
[4]. PS Kumar, AL Griffen, JA Barton, BJ Paster, ML Moeschberger, EJ Leys, New bacterial species associated with chronic periodontitisJ Dent Res 2003 82:338-44. [Google Scholar]
[5]. G Mayanagi, T Sato, H Shimauchi, N Takahashi, Detection frequency of periodontitis associated bacteria by polymerase chain reaction in subgingival and supragingival plaque of periodontitis and healthy subjectsOral Microbiol Immunol. 2004 19:379-85. [Google Scholar]
[6]. PJ Macuch, ACR Tanner, Campylobacter Species in Health, Gingivitis, and Periodontitis. J Dent Res 2000 79:785-92. [Google Scholar]
[7]. H Uematsu, P Nunez, N Sato, F Nakazawa, E Hoshino, Campylobacter rectus and Campylobacter gracilis Isolated from Human Periodontal PocketsJournal of oral Biosciences 2011 53:356-65. [Google Scholar]
[8]. P Williams, E Griffiths, Bacterial transferrin receptors - structure, function and contribution to virulenceMed Microbiol Immunol 1992 181:301-22. [Google Scholar]
[9]. KG Wooldridge, PH Williams, Iron uptake mechanisms of pathogenic bacteriaFEMS Microbiol Rev 1993 12:325-48. [Google Scholar]
[10]. D Grenier, S Tanabe, Transferrin as a source of iron for [10]Campylobacter rectusJournal of Oral Microbiology 2011 3:56-60. [Google Scholar]
[11]. V Goulet, B Britigan, K Nakayama, D Grenier, Cleavage of human transferrin by Porphyromonas gingivalis gingipains promotes growth and formation of hydroxyl radicals.Infect Immun 2004 72:4351-56. [Google Scholar]
[12]. P Duchesne, D Grenier, D Mayrand, Binding and utilization of human transferrin by Prevotella nigrescensInfect Immun 1999 67:576-80. [Google Scholar]
[13]. D Grenier, D Mayrand, Nutritional relationships between oral bacteriaInfect Immun. 1996 53:616-20. [Google Scholar]
[14]. H Ihara, T Miura, T Kato, K Ishihara, T Nakagawa, S Yamada, Detection of Campylobacter rectus in periodontitis sites by monoclonal antibodiesJ Periodont Res 2003 38:64-72. [Google Scholar]
[15]. GC Armitage, Development of a Classification System for Periodontal Diseases and Conditions.Ann Periodontol 1999 4:1-6. [Google Scholar]
[16]. Pelt-Verkuil van, . Elizabeth, Belkum Alex Van, Hays John P, Principles and technical aspects of PCR amplification. India: Springer Science & Business Media, 2008 [Google Scholar]
[17]. S D’Ercole, G Catamo, D Tripodi, R Piccolomini, Comparison of culture methods and multiplex PCR for the detection of periodontopathogenic bacteria in biofilm associated with severe forms of periodontitisNew Microbiologica 2008 31:383-91. [Google Scholar]
[18]. Pointe Scientific [internet].A Med Test Company: Iron/TIBC Reagent Set. 2014. [cited 2014, Feb 23].Available fromhttp://www.pointescientific.com/diagnostic_reagents/product/53. [Google Scholar]
[19]. Transferrin (Immunoturbidimetric Test, 5 + 1 ) [Internet]. 2011[cited 2011 September]. Available fromhttp://www.greiner-diagnostic.com/pdfs/trf.pdf. [Google Scholar]
[20]. F Dati, G Schumann, L Thomas, F Aguzzi, S Baudner, J Bienvenu, Consensus of a group of professional societies and diagnostic companies on guidelines for interim reference ranges for 14 proteins in serum based on the standardization against the IFCC/BCR/CAP reference material (CRM 470).Eur J Clin Chem Clin Biochem 1996 34:517-20. [Google Scholar]
[21]. Y Abiko, T Sato, G Mayanagi, N Takahashi, Profiling of subgingival plaque biofilm microflora from periodontally healthy subjects and from subjects with periodontitis using quantitative real-time PCRJ Periodont Res 2010 45:389-95. [Google Scholar]
[22]. I Saygun, N Nizam, I Keskiner, V Bal, A Kubar, C Ac¸ Ikel, Salivary infectious agents and periodontal disease statusJ Periodont Res 2011 46:235-39. [Google Scholar]
[23]. M Sanz, L Lau, D Herrera, JM Morillo, A Silva, Methods of detection of Actinobacillus actinomycetumcomitans, Porphyromonas gingivalis and Tannerella forsythensis in periodontal microbiology with special emphasis on advanced molecular techniques: a reviewJ Clin Periodontal 2004 31:1034-47. [Google Scholar]
[24]. X Xu, D Kolodrubetz, Construction and analysis of hemin binding protein mutants in the oral pathogen Treponema denticola.Res Microbiol 2002 153:569-77. [Google Scholar]
[25]. E Adonogianaki, J Mooney, DF Kinane, The ability of gingival crevicular fluid acute phase proteins to distinguish healthy, gingivitis and periodontitis sitesJ Clin Periodontol 1992 19:98-102. [Google Scholar]
[26]. P Duchesne, D Grenier, D Mayrand, Binding and utilization of human transferrin by Prevotella nigrescensInfect Immun 1999 67:576-80. [Google Scholar]
[27]. D Grenier, V Goulet, D Mayrand, The capacity of Porphyromonas gingivalis to multiply under iron-limiting conditions correlates with its pathogenicity in an animal model.J Dent Res 2001 80:1678-82. [Google Scholar]
[28]. S Mukherjee, The role of crevicular fluid iron in periodontal diseaseJ Periodontol 1985 56:22-27. [Google Scholar]
[29]. JR Cortelli, DR Aquino, SC Cortelli, CB Fernandes, J de Carvalho-Filho, Nobre Franco GC, et al. Etiological analysis of initial colonization of periodontal pathogens in oral cavityJ Clin Microbiol 2008 46:1322-29. [Google Scholar]
[30]. E Kononen, S Paju, PJ Pussinen, M Hyvonen, P Di Tella, L Suominen-Taipale, Population-based study of salivary carriage of periodontal pathogens in adults.J Clin Microbiol 2007 45:2446-51. [Google Scholar]
[31]. CA Spiegel, G Telford, Isolation of Wolinella recta and Actinomyces viscosus from an actinomycotic chest wall massJ Clin Microbiol 1984 20:1187-89. [Google Scholar]
[32]. XY Han, JJ Tarrand, DC Rice, Oral Campylobacter species involved in extraoral abscess: a report of three cases.J Clin Microbiol 2005 43:2513-15. [Google Scholar]
[33]. Vries De, NLA Arents, WL Manson, Campylobacter species isolated from extra-oro-intestinal abscess: a report of four cases and literature reviewEur J Med Microbiol Infect Dis. 2008 27:1119-23. [Google Scholar]
[34]. SD Mahlen, III Clarridge JE, Oral abscess caused by Campylobacter rectus: case report and literature reviewJ Clin Microbiol 2009 47:848-51. [Google Scholar]
[35]. EJ Goldstein, DM Citron, CV Merriam, YA Warren, K Tyrell, H Fernandez, Comparative invitro activity of ertapenem and other antimicrobial agents against aerobic and anaerobic pathogens isolated from skin and soft tissue animal and human bite wound infectionsJ Antimicrob Chaemother 2001 48:641-51. [Google Scholar]
[36]. JYW Lam, KL Wu Alan, DC Ngai, LL Teng Jade, SY Wong Elsa, KP Lau Susanna, Three cases of severe invasive infections caused by Campylobacter rectus and first report of fatal Crectus infection. J Clin Microbiol 2011 49:1687-91. [Google Scholar]
[37]. RM Arce, PI Diaz, SP Barros, P Galloway, Y Bobetsis, D Threadgill, Characterization of the invasive and inflammatory traits of oral Campylobacter rectus in a murine model of fetoplacental growth restriction and in trophoblast culturesJournal of Reproductive Immunology 2010 84:145-53. [Google Scholar]
[38]. A Yeo, MA Smith, D Lin, EL Riché, A Moore, J Elter, Campylobacter rectus mediates growth restriction in pregnant mice.J Periodontol 2005 76:551-57. [Google Scholar]
[39]. M Yokoyama, D Hinode, M Yoshioka, M Fukui, S Tanabe, D Grenier, Relationship between Campylobacter rectus and periodontal status during pregnancy.Oral Microbiol Immunol 2008 23:55-59. [Google Scholar]
[40]. JW Hutter, U Van der Velden, A Varoufaki, RA Huffels, FJ Hoek, BG Loos, Lower numbers of erythrocytes and lower levels of haemoglobin in periodontitis patients compared to control subjects.J Clin Periodontol 2001 28:930-36. [Google Scholar]
[41]. SR Gokhale, S Sumanth, AM Padhye, Evaluation of blood parameters in patients with chronic periodontitis for signs of anemiaJ Periodontol 2010 81:1202-06. [Google Scholar]