Mucinous Cystadenocarcinoma Co-Existing with Mature Cystic Teratoma : A Rare Case Report
Prachi Kukreja1, Sunil Kumar Yeshvanth2, Teerthanath Shrinivas3, Tanu Agrawal4, Jayaprakash K Shetty5
1Postgraduate, Department of Pathology, KS Hegde Medical Academy, Mangalore, India.
2Associate Professor, Department of Pathology, KS Hegde Medical Academy, Mangalore, India.
3Professor, Department of Pathology, KS Hegde Medical Academy, Mangalore, India.
4Postgraduate, Department of Pathology, KS Hegde Medical Academy, Mangalore, India.
5Professor and Head, Department of Pathology, KS Hegde Medical Academy, Mangalore, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Prachi Kukreja, Postgraduate, Department of Pathology, KS Hegde Medical Academy, Nithyananda Nagar Post, Deralakatte, Mangalore- 575018, India.
E-mail: prachikukreja@gmail.com
Co-existence of mucinous cystadenocarcinoma with mature cystic teratoma in the same ovary is very rare. We report a case in a 48-year-old female who presented with left abdominal mass. CT scan revealed a heterogeneous enhancing left ovarian mass lesion. Clinical diagnosis of complex ovarian cyst was made, later underwent laparotomy and histologically diagnosed as mucinous cystadenocarcinoma (grade 2) co-existing with benign cystic teratoma, stage Ia (FIGO) of the left ovary. Six months after surgery, the patient is doing well without any recurrence or metastasis. Hence, histopathological examination plays a significant role in accurate diagnosis and management of the patient. So, we should be aware of these rare co-existent tumours and meticulous dissection should be done to look for any synchronous tumours or malignant areas; since management and prognosis will vary significantly depending upon the microscopic type and stage.
Cyst ovary, Dermoid, Mucinous neoplasm
Case Report
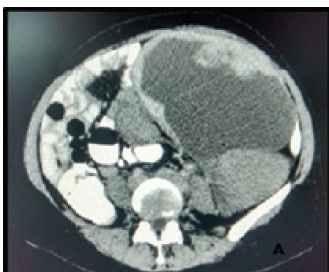
A 48-year-old female (P1L1) came to Obstetrics and Gynaecology OPD of K.S Hegde Charitable Hospital, Mangalore, India with history of pain abdomen which was dull aching and non radiating. There were no history of dysmenorrhoea, menorrhagia, burning micturition, bowel and bladder disturbances.With proper patient consent, per abdomen examination revealed a nodular; soft to firm ill defined mass measuring 10x5 cm was present in the left iliac fossa reaching the hypogastrium. CT pelvis showed a left ovarian mass lesion with predominantly cystic with focal solid areas [Table/Fig-1a]. Uterus, right ovary and other abdominal organs were normal. A clinical diagnosis of complex ovarian cyst was made. CA-125 was 52.5 U/ml (normal value <35U/ml). Other routine biochemical and haematological parameters were within normal limits. Laparotomy was performed and entire specimen was sent to pathology department for histopathological examination. Postoperative period was uneventful. The patient on follow up is doing well without any recurrence or metastasis as of six months after surgery.
Grossly, we received a specimen of total abdominal hysterectomy with bilateral salpingo-oophorectomy, omental tissue and left and right iliac lymph nodes. The left ovarian tumour measured 16x9x6.5 cm. Cut section revealed well encapsulated, multiloculated cystic tumour with focal solid areas [Table/Fig-1b]. Cystic areas were filled with mucinous material and solid areas were grey white with focal papillary excrescences. Uterus, cervix, left fallopian tube, right adnexa and omental tissue were grossly unremarkable.
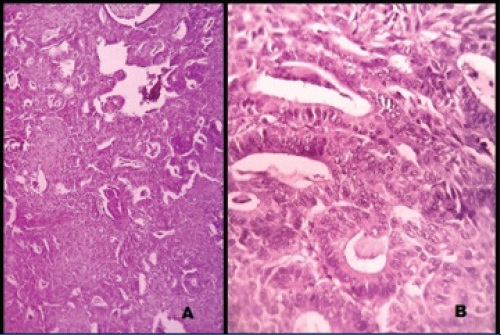
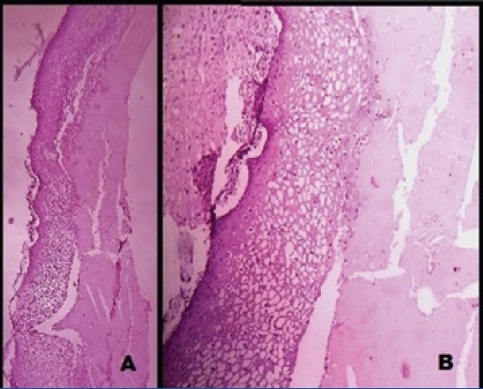
Microscopically, solid areas showed proliferating glands lined by tall columnar cells having pleomorphic nuclei with stratification, prominent nucleoli and hyperchromatism [Table/Fig-2a,b]. Plenty of intracellular mucin was also seen. Focal areas showed cyst wall lined by stratified squamous epithelium associated with osteoid and keratinous material [Table/Fig-3a,b]. No evidence of immature teratoma component was seen. Sections from the capsule showed no tumour infiltration. Omentum, right ovary, uterus, cervix, right and left iliac lymph nodes were free from the tumour. The final diagnosis of mucinous cystadenocarcinoma (grade 2) co-existing with mature cystic teratoma, stage Ia (FIGO) of the left ovary was made.
Discussion
Mucinous cystadenocarcinomas are only one-third as common as serous cystadenocarcinomas and occur in the age group of 45-65 years. They are typically large, unilateral, multilocular cystic masses containing watery or viscid secretions with smooth white capsules and have average size of 18-22 cm. They may have solid areas with foci of haemorrhage and necrosis [1-3]. Microscopically they show complex papillary areas or back to back glands lined by pleomorphic cells with stromal invasion. Mucinous cystadenocarcinomas may arise de novo or transformation from a benign mucinous cystadenoma which is estimated around 12% [2,3]. Approximately 70% of these patients present with advanced stages i.e. would have metastasised to upper abdomen and beyond abdominal cavity because these patients mistake the initial symptoms as other minor GIT disorders.
Mature cystic teratoma of the ovary (dermoid cyst) comprises 27%-44% of all ovarian tumours. It is the most common germ cell neoplasm occurring in reproductive age group and usually presents with a mass, but 25% are detected incidentally. It presents as multiloculated masses and contain cheese like cystic component composed of keratin, sebum and hair [2,3].
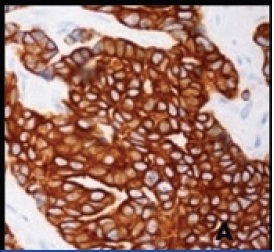
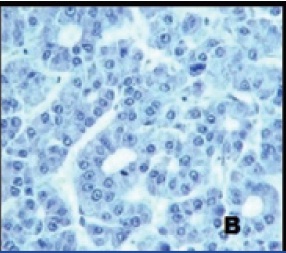
Mucinous tumours can rarely co-exist with other tumours like mature cystic teratoma, serous tumours, Brenner tumours, thecoma etc. The incidence of mucinous tumours co-existing with teratoma is just 3%-8% [4]. However, mucinous tumours can be surface epithelial origin or teratomatous origin. In our case, since mucinous cystadenocarcinoma component is a predominant tumour with only focal teratomatous component, we made a diagnosis of coexistence of these tumours. However, immunohistochemistry helps to differentiate the exact origin of mucinous tumours. CK 7 is positive and CK 20 negative in surface epithelial origin; whereas CK7 is negative and CK 20 positive in teratomatous origin [4-8]. Immunohistochemistry in our case revealed strong membrane positivity for CK7 and negativity for CK 20, hence confirming the histogenesis of mucinous cystadenocarcinoma from surface epithelium of the ovary [Table/Fig-4a,4b].
On reviewing literature, we found Okada et al., in 2004 reported only 2 cases (out of 11) of mucinous cystadenocarcinoma coexisting with mature cystic teratoma [9]. Russel Vang et al., reported 44 cases of ovarian mucinous tumours associated with mature cystic teratoma out of which six were malignant [4]. Jesse K Mc Kenney et al., reported 5 out of 42 cases of ovarian mucinous cystadenocarcinomas associated with mature cystic teratoma [5].
Contrast enhanced CT pelvis showing a left ovarian mass lesion with predominantly cystic with focal solid areas
Gross photograph of the ovarian tumour showing encapsulated, multiloculated, predominantly cystic, filled with thick mucinous fluid and foci of solid areas (arrow)

Microscopic view of the solid area showing mucinous
adenocarcinoma displaying proliferating glands with intraglandular mucin lined by tall columnar cells having pleomorphic nuclei with stratification, prominent nucleoli and hyperchromatism (2a) H&E x 100X, (2b) H&E x 400X
Microscopic view of the focal cystic area showing mature cystic teratoma displaying stratified squamous epithelial lining (3a) H&E x 100X, (3b) H&E x 400X
Immunohistochemistry revealing strong CK7 membrane positivity
Immunohistochemistry revealing CK20 negativity
Conclusion
Majority of these studies and our experience showed that, the distinction between benign, borderline, and malignant tumour are generally not possible by radiological studies and only diagnosed by histopathological examination. Hence, histopathological examination and immunohistochemistry plays a significant role in accurate diagnosis and management of these patients. So, we should be aware of these rare co-existent tumours and meticulous dissection should be done to look for any synchronous tumours or malignant areas; since management and prognosis vary significantly depending upon the microscopic type and stage.
[1]. JD Seidman, KR Cho, BM Ronnett, RJ Kurman, Surface epithelial tumours of the ovary. In: Kurman RJ, Ellenson LH, Ronnett BM, editors.Blaustein’s pathology of female genital tract. 2011 6th EditionLondonSpringer:679-784. [Google Scholar]
[2]. FA Tavassoli, P Devilee, editors. World Health Organisation classification of tumours, pathology and genetics, tumours of the breast and female genital organs. Lyon: IARC Press 2003 [Google Scholar]
[3]. S Ray, D Anuradha, G Barui, R Karmakar, A Sinha, A Bhattacharya, Coexistence of a mature teratoma and mucinous cystadenocarcinoma in the same ovary-a case report.Indian J PatholMicrobiol 2006 49:420-22. [Google Scholar]
[4]. R Vang, AM Gown, C Zhao, TS Barry, C Isacson, MS Richardson, Ovarian mucinous tumours associated with mature cystic teratomas: Morphologic and immunohistochemical analysis identifies a subset of potential teratomatous origin that shares features of lower gastrointestinal tract mucinous tumours more commonly encountered as secondary tumours in the ovaryAm J Surg Pathol 2007 31:854-69. [Google Scholar]
[5]. JK McKenney, RA Soslow, TA Longacre, Ovarian mature teratomas with mucinous epithelial neoplasms: morphologic heterogeneity and association with pseudomyxomaperitonei.Am J SurgPathol 2008 32:645-55. [Google Scholar]
[6]. K Fugii, Y Yamashita, T Yamamoto, K Takahashi, K Hashimoto, T Miyata, Ovarian mucinous tumours arising from mature cystic teratomas- a molecular genetic approach for understanding the cellular originHum Pathol 2014 45:717-24. [Google Scholar]
[7]. K Kajo, L Masak, D Sorkovska, M Vallova, M Kajo, K Machalekova, Mucinous carcinoma (non-intestinal type) arising in the ovarian mature cystic teratoma - a case reportCesk Patol 2013 49:141-45. [Google Scholar]
[8]. JH Park, SO Whang, ES Song, SJ Choi, WY Lee, An ovarian mucinous cystadenocarcinoma arising from mature cystic teratoma with para-aortic lymph node metastasis: a case report.J GynecolOncol 2008 19:275-78. [Google Scholar]
[9]. S Okada, Y Ohaki, J Ogura, M Ishihara, T Kawamura, T Kumazaki, Computed tomography and magnetic resonance imaging findings in cases of dermoid cystco-existing with surface epithelial tumours in the same ovary.J Comput Assist Tomogr. 2004 28:169-73. [Google Scholar]