Hirschsprung’s disease is the commonest cause of neonatal intestinal obstruction [1]. Approximately 80% of the patients are male [2]. Harald Hirschsprung described this condition as congenital megacolon in 1886 [3,4]. Hirschsprung’s disease is currently regarded as a genetic disorder with a complex pattern of inheritance. RET proto-oncogene apparently playing a major role. 10% of the neonates are seen to have Down’s syndrome and another 5% have other neurological abnormalities [5].
The diagnosis is based on histopathological analysis of rectal biopsies that sample mucosa and underlying submucosa [6]. Two different approaches have evolved to identify ganglion cells. The first, which is used in most paediatric pathology laboratories, is to evaluate numerous H&E stained levels from each paraffin embedded biopsy [7]. The reliability of this method depends on the observer’s ability to accurately distinguish a ganglion cell based on its H&E.
Calretinin is a vitamin D dependent calcium-binding protein involved in calcium signaling. In rectal biopsies that contain ganglion cells, small nerves in the lamina propia, muscularis mucosae, and superficial sub mucosa contain granular aggregates of calretinin immunoreactivity [8]. Immunolabelling of these nerves was completely absent in the aganglionic biopsies of Hirschsprung’s disease patients. Calretinin immunohistochemistry yielded no misdiagnoses or major discrepancies between observers. In our study we have attempted to find out the value of this novel method in diagnosis of Hirschsprung’s disease and compare the same with the conventional histopathology.
Materials and Methods
Case selection
This prospective observational study was carried out in the Department of Pathology and Department of Paediatric Surgery from July 2013 to September 2014. Institutional Ethics committee approval was taken. Eighty nine neonates (age range- 0- 28 days) with clinically suspected Hirschsprung’s disease were selected. Cases with inadequate specimens were not included in this study. After taking valid consent from the parents a detailed history was taken and clinical examination along with any presence of malformation was noted preoperatively.
Histopathological examination
The full thickness biopsy specimens were sent for histopathological examination. One hundred ninety six blocks (168 from biopsies and 28 from total colectomy specimens) were sectioned and stained with haematoxylin & eosin staining. Representative sections from spastic segment, transitional zone and dilated segment were embedded. All slides were examined under light microscope by two observers. One distal colon full thickness sample and one proximal colon full thickness sample with microscopic evidence of ganglion cells were taken as positive controls. The presence of ganglion cells was confirmed with S-100 immunohistochemistry in positive controls. One slide from spastic segment of previously diagnosed case of Hirschsprung’s disease was taken as negative control.
Immunohistochemistry
Ninety six blocks were selected for immunohistochemical staining. Blocks without representative tissue or containing only superficial mucosa were eliminated. 3 μm sections were taken on poly-l –lysine coated slides. Ready-to-use Calretinin from cell Dako (FLEX Monoclonal Mouse Anti- Human Calretinin Clone DAK- Calret 1) was used for immunostaining. The antibody used predominantly stained cytoplasm and nuclei of ganglion cells. Calretinin was considered as positive if any specific staining was present either within the sub mucosal or the myenetric plexus. Mast cell staining was considered as internal positive control.
S-100 staining was done in positive control with ready to use S-100 from Dako (FLEX Polyclonal Rabbit Anti S-100) and brown cytoplasm and nuclear staining of the ganglion cells were considered as positive [9].
Statistical Analysis
Histopathological diagnosis were taken as the ‘gold standard’ and the other procedures were statistically analysed using Chi-Square test, Sensitivity, Specificity, Positive predictive value, Negative predictive value and Diagnostic accuracy etc. Software used in statistical analysis of our study was MedCalc version 11.6 (Mariakerke, Belgium: MedCalc Software 2011). Agreement between two observers was analysed.
Results
Clinical profile
A total no of 89 cases were included in this study of which 69 patients were male (77.52%) and rest 20 patients (22.48%) were female [Table/Fig-1]. The age ranged was from 0 days to 28 days.
Graph showing distribution of age and sex in studied patients
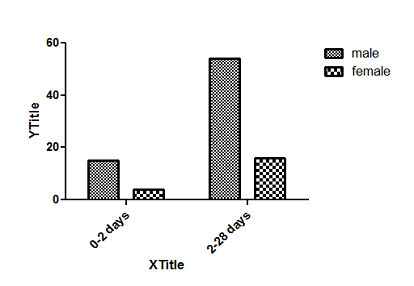
[Table/Fig-2] Distribution of age and sex in studied patients. Delayed passage of meconium (38.3%) was the main diagnostic symptom in most cases, followed by abdominal distension (32.6%). Other presenting symptoms were gastrointestinal perforation, bilious vomiting and meconium ileus [Table/Fig-3].
Distribution of age and sex in studied patients
| MALE | FEMALE |
|---|
| AGE | NUMBER | PERCENTAGE | NUMBER | PERCENTAGE |
|---|
| 0-2 DAYS | 15 | 21.7% | 4 | 20% |
| 2-28 DAYS | 54 | 78.3% | 16 | 80% |
Clinical manifestation of patients in hirschsprungs’ disease
| SYMPTOMS AND SIGNS | NUMBERS | % |
|---|
| ABDOMINAL DISTENTION | 29 | 32.6% |
| GI PERFORATION | 18 | 20.2% |
| DELAYED PASSAGE OF MECONIUM | 34 | 38.3% |
| BILIOUS VOMITING | 6 | 6.7% |
| MECONIUM ILEUS | 2 | 2.2% |
[Table/Fig-4] Clinical manifestation of patients in hirshprungs’ disease.
Neonates having down syndrome suffering from hirschprung's disease
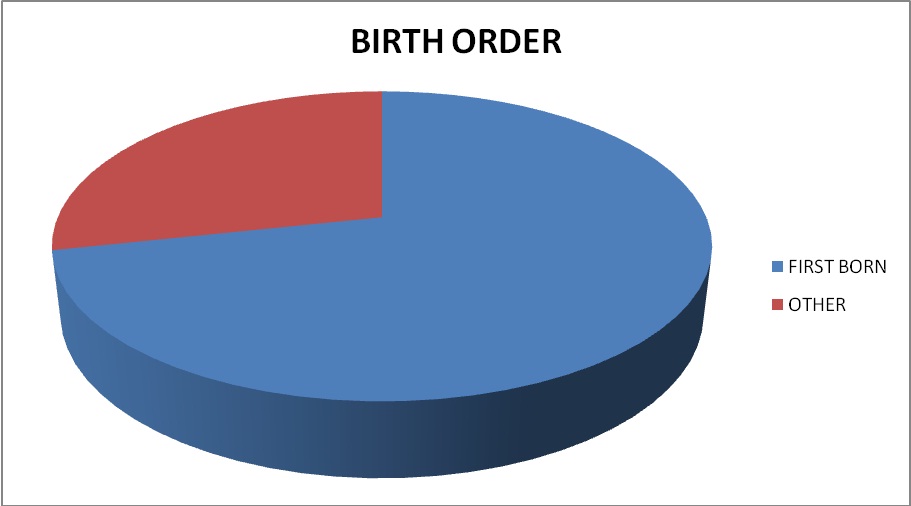
We also observed a number of associated anomalies in cases of suspected Hirschsprung’s disease. The commonest genetic disorder in our study was trisomy 21 (Down’s syndrome) (30%) followed by Waardenburg-Shah syndrome and Joubert’s syndrome [Table/Fig-5]. Birth order of the patients was documented and analysed [Table/Fig-6].
Associated syndromes and malformation
| MALFORMATION | NUMBER OF CASES | PERCENTAGE |
|---|
| DOWN SYNDROME( TRISOMY 21) | 27 | 30 |
| WAARDENBURG-SHAH SYNDROME | 1 | 1.2 |
| JOUBERT’S SYNDROME | 1 | 1.2 |
| TETRALOGY OF FALLOTS | 1 | 1.2 |
| ANORECTAL MALFORMATION | 1 | 1.2 |
| IMPERFORATE ANUS | 2 | 2.25 |
| MULTIPLE INTESTINAL ATRESIA | 2 | 2.25 |
| INTESTINAL MALROTATION | 1 | 1.2 |
Percentage of patients according to birth order
Histopathological examination
To define the site of involvement in hirshprungs’ disease, we subdivided as follows: 1) rectosigmoid (short segment); 2) long segment; 3) ultra short segment; 4) total colonic and 5) total intestinal. [Table/Fig-7] shows that the most common site of involvement was rectosigmoid region (78.6%).
Distribution of different types of involved segment
| LENGTH OF BOWEL INVOLVED | NUMBER OF CASES | % |
|---|
| Rectosigmoid(Short Segment) | 70 | 78.6% |
| Total colonic | 7 | 7.9% |
| Total intestinal | 0 | 0 |
| Long segment | 11 | 12.4% |
| Ultrashort segment | 1` | 1.1% |
| Total | 89 | 100 |
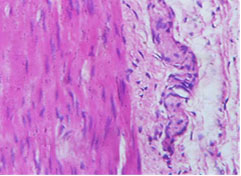
Regarding histopathological examination, two observers separately examined 196 slides from 89 neonates. Microscopically, slides from 77 patients out of 89 neonates show absence of ganglion cells and considered as Hirshprungs’ disease by both observers. Only one case regarded negative by one observer but considered positive by other pathologist. Eleven patients show presence of ganglion cells [Table/Fig-8,9]. [Table/Fig-10] demonstrate high consensus between two pathologists (p<0.001).
Mature ganglion cells in myenteric plexus (400X)
Hypertrophic nerve fibres (400X)
Observer’s evaluation in H&E stained smears
| 1st observer | 2nd observer | Number of cases |
|---|
| Ganglion cell absent | Ganglion cell absent | 77/89(91%) |
| Ganglion cell absent | Ganglion cell present | 1/89(1.1%) |
| Ganglion cell present | Ganglion cell absent | 0/89(0) |
| Ganglion cell present | Ganglion cell present | 11/89(7.9%) |
The two sided p-value is < 0.0001, considered extremely significant. The row/ column association is statistically significant
IHC profiling of calretinin
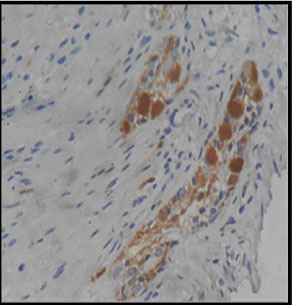
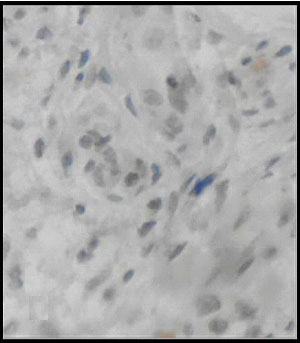
Ninety six blocks were selected for calretinin immunohistochemistry. All representative areas including lamina propria, submucosa and muscularis mucosae were examined for detection of calretinin positivity by two pathologists blindly. Satisfactory concordance was obtained between H&E reports and calretinin study. Overall sensitivity, specificity, positive and negative predictive value were 100 %, 97.44%, 84.62% and 100% respectively [Table/Fig-11]. Only one case reported as hirshprungs’ disease by both pathologists, show calretinin positivity in muscularis mucosae [Table/Fig-12,13].
Detection of Ganglion Cells by Routine H&E Staining and Calretinin Immunohistochemical Analysis in 89 Patients Suspected of Having Hirschsprung Disease
| Total | H&E | CALRETININ | MM(s) | MM | SM(s) | SM | MP | MP(O) |
|---|
| 11 | + | + | 0 | 2 | 0 | 0 | 9 | 0 |
| 02 | _ | + | 0 | 2 | 0 | 0 | 0 | 0 |
| 00 | + | _ | 0 | 0 | 0 | 0 | 0 | 0 |
| 76 | _ | _ | 0 | 25 | 0 | 0 | 51 | 0 |
MM- biopsy specimens containing mucosa and muscularis mucosae; MM(s)- mucosa and superficial muscularis mucosae; MP-mucosa, muscularis mucosae, submucosa, and muscularis propria; MP(o)- muscularis propria only; SM- mucosa, muscularis mucosae, and submucosa; SM(s)-mucosa, muscularis mucosae, and superficial submucosa The two sided p-value is < 0.0001, considered extremely significant. The row/ column association is statistically significant
Immunohistochemistry showing calretinine positivity (400X)
Immunohistochemistry showing calretinine negativity (400X)
Discussion
In present study clinico-morphological profile and histopathological examination was done in all cases of suspected Hirschprung’s disease. Combination of clinical, radiological and histopathological examination is necessary for establishment of diagnosis of Hirschprung’s disease. According to Dalla–Valle, aganglionosis in involved segment is the characteristic feature of Hirschprung’s disease [10,11]. In case of neonates, identification of immature ganglion cells in submucosal and myenteric plexus is difficult in routine H&E stain [12]. Introduction of enzyme histochemistry and immunohistochemistry helps in reduction of false positive cases of Hirschprung’s disease [13,14].
Introduction of histochemical method using acetyl cholinesterase was considered as novel method in the past. Colonic aganglionosis along with high acetyl cholinesterase is diagnostic hallmark. Requirement of fresh frozen section and high level of expertise are the main disadvantages. As per as the neonatal age group is concerned, enzyme system is immature and muscularis mucosa is not well developed. In neonates specially within first 3 weeks of life, an increase in AChE reaction is not detected in patients of Hirschprung’s disease [15]. Due to this drawback, requirement of more specific neural marker is considered. In numerous studies compare the results of several immunohistochemical markers like S-100, NSE, GFAP, GLUT-1 and MAP-5 [16–20].
We select calretinin, a general marker of peripheral nervous system in adjunct to routine H&E stain. Qualitative evaluation of calretinin immunostaining is simple and valuable tool for detection of Hirschprung’s disease [21]. Negative immunohistochemical expression calretinin in both submucosal and myenteric plexus of spastic segment of colon is diagnostic [22].
Overall sensitivity, specificity, positive and negative predictive value were 100%, 97.44%, 84.62% and 100% respectively. Our finding was concordant with other studies [23,24].
Conclusion
Qualitative interpretation of Calretinin immunohistochemistry is considered as a superior diagnostic tool in Hirschsprung’s disease especially in neonates.