Case Report
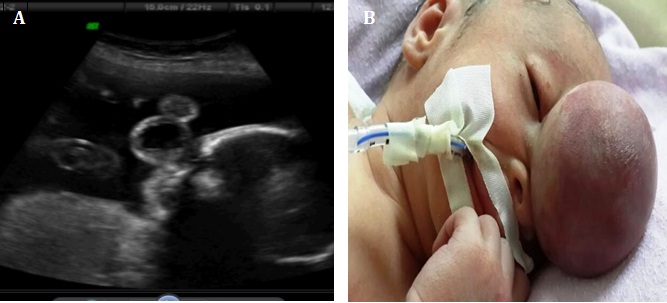
A 24-year-old late registrant admitted to the Perinatology Clinic of Zekai Tahir Burak Women's Health Education and Research Hospital for the first time at 18 weeks of gestation. Her past medical history was unremarkable. The routine examination with two-dimensional sonography (USG) (Voluson E730; GE Healthcare) revealed a singleton fetus, appropriate for gestational age biometry with overall normal anatomy other than a midline soft-tissue mass which contained brain tissue and cerebrospinal fluid measuring 1.8 × 1.5 × 1.3 mm in size, with a cyst in a cyst appearance, projecting anteriorly through a bony defect, from the lower part of the forehead between the orbits [Table/Fig-1a]. The posterior fossa contents appeared normal and no other abnormalities were noted in the detailed USG.The parents, following counseling about the status of the fetus, declined both termination of the pregnancy and further genetic evaluation.
The progress of the pregnancy was uneventfull until the 35th week when the patient came to our emergency unit with vaginal bleeding and abdominal pain. A live baby boy weighing 2140 gm with a head circumference of 34.5 cm was delivered by caesarean section. The baby was noted to have a 5x4cm midline soft tissue mass in the forehead associated with hypertelorism and a wide nose bridge. The overlying skin was intact. No other systemic abnormalities were detected [Table/Fig-1b].
The magnetic resonance imaging (MRI) scan, confirming the bone defect between the frontal and nasal bones, the herniation of the meninges, cerebrospinal fluid (CSF), and the brain tissue, also revealed concurrent deformation of the inferior frontal gyrus, slight dilatation of the lateral ventricles and spina bifida occulta at the 1st sacral level. The rest of the intracranial anatomy was normal. A one-stage operation was carried out when the baby was 7-days-old. The exploration of the sac and excision of the malformed brain tissue was followed by reconstruction of the dural defect by synthetic graft, muscle and fibrin glue. Postoperative minor cerebrospinal fluid leakage from the wound eventually stopped spontaneously. Histopathologic examination of the excised brain tissue revealed dysplastic, gliotic structure. On the day of surgery, pallor and swelling was noticed in the right lower limb. Screening for genetic procoagulant mutations revealed combined heterozygous MTHFR 677C-T and PAI-1 4G/5G gene mutations. Upon progression of ischemic skin changes in the right leg and left foot in the following days, as the vascular surgeons suggested, anticoagulation was started and the umbilical catheter was removed. Despite conservative management, at the age of 34 days, he underwent right lower limb amputation above the knee with the diagnosis of arterial thrombosis [Table/Fig-2].
The additional MRI scans of the brain and cervical spine of the neonate within the following several neonatal days, in contrast to the first postpartum scan, demonstrated partial elevation of cerebellar tonsils, the posterior fossa appearances resembling clearly those of the Chiari I anomaly, corpus callosum agenesis and progressive dilatation of the lateral ventricles.
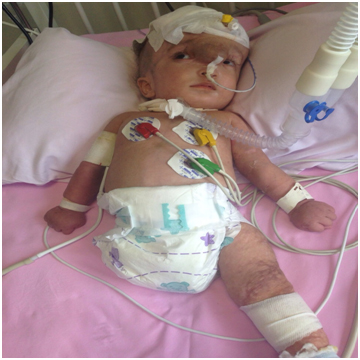
Initial blood parameters and the biochemical tests were all within normal range for a newborn. Polyuria and hypernatremia (152 mmol/L), associated serum hyperosmolality (327 mOsm/kg) and low urine osmolality (180 mOsm/kg), made us think of the presence of central diabetes insipidus. While the serum growth hormone (7.8 ng/ml) and insulin (2 mU/L) levels were within the normal range, the cortisol (0,74 g/dL) was lower than normal, compatible with adrenal insufficiency. Desmopressin and cortisone treatment were started. The child (now almost 8 months old), shows normal growth spurts, is neurologically normal but still with hydrocephalus and the protruding frontal sac, hospitalized currently, with respiratory distress due to pneumonia, is kept on anticoagulation, steroid and desmopressin treatments.
Informed consent: Informed consent was obtained from the patient.
Discussion
Encephalocele is a rare congenital defect (one in 13,000 births) characterized by herniation of cranial contents through a defect in the skull [1]. While most encephaloceles occur at the occipital area, the frontal ones are less common with an incidence of 1 per 40,000 live births. Of infants with encephalocele, 15% have associated neural tube defects and 40% have additional anomalies [2].
Maternal diabetes, rubella, hypervitaminosis-A, amniotic band syndrome, aneuploidy including trisomies 13 and 18, and genetic syndromes such as Dandy-Walker and Meckel-Gruber have been suggested as potential causes of encephaloceles. Due to the increased incidence of chromosomal abnormalities coexisting with encephalocele, a prenatal karyotype should be offered [3-5]. Despite improvements in surgical techniques, overall morbidity and mortality is still high [1,2].
In the presented case, although early postpartum imaging demonstrated the cerebellar tonsils in the posterior fossa, Chiari I malformation was diagnosed in the following weeks. Despite the traditional concept of Chiari I malformation’s being a congenital abnormality, evidence has accumulated in recent years, to suggest that cerebellar tonsillar herniation may be an acquired phenomenon [6].
Symptomatic neonatal thromboembolic disease (TE) is a rare condition with an incidence of 0.51 per 10,000 live births [7,8]. Neonatal thrombosis should be differentiated from intrauterine thrombosis. Arterial thromboses which account for 50% of all thrombotic incidents in neonates, occur almost completely as iatrogenic complications of arterial appliances including peripheral or umbilical arterial catheters [9]. Although most of the arterial TEs are asymptomatic, large thrombosis can present with necrotising enterocolitis, renal failure and peripheral limb ischaemia due to occlusions and embolic incidents [6,8].
The presented case who revealed signs of ischemia in the lower extremities on the day of encephalocele surgery, ended up with limb amputation despite conservative management including immediate removal of umblical artery catheter and anti-coagulation treatment. Nevertheless, thrombophilic conditions were screened in our case and we detected two genetic prothrombotic risk factors, heterozygous MTHFR and 4G/5G mutations. Although it is difficult to claim that the aetiology of arterial thrombosis in our case is the genetic mutations alone, we believe that the presence of a combination of both PAI-1 and MTHFR might cause a potential risk for the occurrence of arterial ischemia.
A significant number of patients with frontal encephalocele present clinical evidences suggestive of hypothalamopituitary insufficiency.
Although aetiology is not known, a possible cause of progressive hormonal dysfunction could be the stretched pituitary stalk and hypothalamus [7,9]. Although our case had normal thyroid function and growth hormone levels, he revealed central hypoadrenalism and central diabetes insipidus, in the early neonatal period.
Ultrasonography is essential to diagnose the fetus for skin covered or closed defects such as the case with encephalocele presented in this report, that will not be identified by maternal serum alpha-fetoprotein screening [10]. The sonographic appearance, the natural history and clinical prognosis depends on the localization and content of the protruded sac and also the presence of the associated anomalies. The most important prognostic factor is the presence of gross brain tissue in the sac [5,10].
In relation to the neonatal management of encephalocele cases, the surgical challenges include closing the anatomical defect in the cranial vault and achieving as near normal functional outcome as possible with minimal psychomotor defects. Although frontal encephaloceles have a better prognosis because the defects are in general smaller the mortality rate is still high [10,11] and the long-term outcome is generally poor. In a study, of the 8 liveborn infants out of 15, only 5 survived beyond the neonatal period, and all of the 3 for whom follow-up information was available had developmental delay [12].
Termination should be discussed with the parents. The parents should be informed about possible developmental defects in the baby. If a large amount of neural tissue is detected in the sac, or if associated anomalies are present, for minimizing maternal risk cesarean section may be considered [13]. Regarding the future reproductive outcome, counselling the couple should include the information that isolated encephalocele is not considered to have an increased risk of recurrence.
a) The ultrasound image which shows the mass measuring 1.8 × 1.5 × 1.3 cm in size (cyst within a cyst appearance), protruding between the frontal and nasal bones at 18th weeks of pregnancy. b) The neonate with the nasofrontal encephalocele at birth
The recent picture of the case at 8 months of age, hospitalized due to pneumonia
Conclusion
We hereby, presented a rare case of prenatally diagnosed frontonasal encephalocele along with several associated abnormalities in CNS and serious complications in the early neonatal period. The presented case was still live at the time of writing of this report but with several severe co-morbidities including loss of extremities and respiratory distress. Once the encephalocele has been verified, we suggest a detailed and careful evaluation to be done for associated anomalies including a prenatal karyotype and also considering the imminent poor prognosis, counseling the parents for termination of pregnancy.
[1]. WJ Whatmore, Sincipital encephalomeningocelesBr J Surg 1973 60:261-70. [Google Scholar]
[2]. B Kälién, E Robert, J Harris, Associated malformations in infants and fetuses with [2]upper or lower neural tube defectsTeratology. 1998 57:56-63. [Google Scholar]
[3]. B Christensen, DS Rosenblatt, Effects of folate deficiency on embryonic development.Baillieres Clin Haematol 1995 8:617-37. [Google Scholar]
[4]. AC Esmer, I Kalelioğlu, H Kayserili, A Yüksel, R Has, Prenatal diagnosis of frontonasal dysplasia with anterior encephaloceleJ Turk Ger Gynecol Assoc 2013 14:50-52. [Google Scholar]
[5]. M Tirumandas, A Sharma, I Gbenimacho, MM Shoja, RS Tubbs, WJ Oakes, Nasal encephaloceles: a review of aetiology, pathophysiology, clinical presentations, diagnosis, treatment, and complicationsChilds Nerv Syst 2013 29:739-44. [Google Scholar]
[6]. A Greenwaya, MP Massicotteb, P Monagle, Neonatal thrombosis and its treatment.Blood Reviews 2004 18:75-84. [Google Scholar]
[7]. S Wacharasindhu, U Asawutmangkul, S Srivuthana, Endocrine abnormalities in patients with frontoethmoidal encephalomeningocele. A preliminary studyHorm Res 2005 64:64-67.. [Google Scholar]
[8]. A Veldman, MF Nold, I Michel-Behnke, Thrombosis in the critically ill neonate:incidence, diagnosis, and management.Vasc Health Risk Manag 2008 4:1337-48. [Google Scholar]
[9]. B Atasay, M Berberoğlu, A Günlemez, O Evliyaoğlu, P Adiyaman, S Unal, Management of central diabetes insipidus with oral desmopressin in a premature neonateJ Pediatr Endocrinol Metab 2004 17:227-30. [Google Scholar]
[10]. NE Budorick, DH Pretorius, JP McGahan, MR Grafe, HE James, J Slivka, Cephalocele detection in utero: sonographic and clinical features.Ultrasound Obstet Gynecol 1995 5:77-85. [Google Scholar]
[11]. CJ Bui, RS Tubbs, CN Shannon, L Acakpo-Satchivi, 3rd Wellons JC, JP Blount, Institutional experience with cranial vault encephalocelesJ Neurosurg 2007 107:22-25. [Google Scholar]
[12]. B Chowchuen, C Thanapaisal, P Chowchuen, P Duangthongpon, Frontoethmoidal meningoencephalocele: challenges and the Tawanchai center's long-term integrated management.J Med Assoc Thai 2011 94:129-40. [Google Scholar]
[13]. MS Chatterjee, B Bondoc, A Adhate, Prenatal diagnosis of occipital encephaloceleAm J Obstet Gynecol 1985 153:646-47. [Google Scholar]