Introduction
Community Acquired Pneumonia (CAP) is one of the commonest causes of patient’s visit to the Emergency Room (ER). Hospitalisation of patient depends on severity of pneumonia. Various pneumonia severity assessment scores are available to predict mortality in community acquired pneumonia but these scores are not commonly used. Procalcitonin is a biomarker which is raised in bacterial infection and is easy and quick to measure. The aim of our study was to assess the ability of baseline serum procalcitonin level to predict mortality of community acquired bacterial pneumonia compared to PSI, CURB-65 and CRB-65 and its add-on value to the simple CRB-65 score.
Materials and Methods
Fifty five patients admitted with Com-munity Acquired Bacterial Pneumonia were enrolled after taking informed consent and satisfying all inclusion and exclusion criteria. PSI, CURB -65, CRB-65 and PCT scores were determined on admission. PCT was measured by semi- quantitative assay; PCT Q. Primary outcome was 30 day mortality. Sensitivity, specificity, positive and negative predictive value of PCT for assessing mortality was calculated and compared to validated pneumonia severity scores; PSI, CURB-65 and CRB-65. We also assessed the ability of the combination of PCT to each of the scores to predict 30 day pneumonia specific mortality.
Results
In receiver operating characteristic analysis for mortality prediction, area under curve (95% CI) for PCT, PSI, CURB-65 and CRB-65 was 0.92 (0.85, 1.0), 0.88 (0.78, 0.98), 0.88 (0.76, 0.99), 0.9 (0.78, 1.0) respectively. Combination of PCT to each of the scores improved the prognostic ability to predict 30 day pneumonia specific mortality.
Conclusion
Semi-quantitative PCT level at admission is an excellent test to predict the outcome of pneumonia. It predicts patients at low risk of mortality from community acquired bacterial pneumonia.
Introduction
Pneumonia is a common cause of infection related mortality worldwide and because of lack of epidemiological surveys; a clear population based statistics is not available. The Center for Disease Control and Prevention combines data of pneumonia and influenza, hence data about pneumonia alone is not available [1]. Lower respiratory tract infections (LRTI) caused 4.2 million deaths (7.1% of all deaths) in 2004 globally; predominantly in the lower and middle income countries. In India, in 2004, the mortality due to LRTI’s was 646000 adult males (> 15 yrs of age) and 574000 adult females (> 15 yrs of age) [2]. Fine reported that in 2006 there were 4 million adults affected with community acquired pneumonia in US, of which upto 20% required hospitalization [3].
Guidelines for management of adult community acquired pneumonia (CAP) recommend a severity based approach. Severity of pneumonia varies from mild to life threatening; hence severity assessment of CAP at presentation is important. Various pneumonia severity assessment scores are developed to objectively assess the severity and guide decision about treatment settings [4,5]. The three most widely validated pneumonia severity assessment scores are the PORT PSI (Pneumonia Outcome Research Team, Pneumonia Severity Index) [6], CURB-65( confusion, urea >7mmol/l, respiratory rate ≥ 30 breaths /min, systolic blood pressure < 90mmHg or diastolic blood pressure ≤ 60 mmHg and age ≥ 65 years) and CRB-65 (confusion, respiratory rate ≥ 30 breaths /min, systolic blood pressure < 90mmHg or diastolic blood pressure ≤ 60 mmHg and age ≥ 65 years) [7]. However these scores are not widely used by clinicians [8–11]. Each score has its various strengths and weaknesses. Moreover there is neither uniform agreement on optimum severity assessment tool nor an agreed definition for severe pneumonia [12].
The ideal severity assessment score would be one which provides a high sensitivity and specificity for predicting severity of illness at baseline without relying on too many laboratory investigations. It also needs to be simple enough to use. Many biomarkers like pro adrenomedullin, atrial natriuretic peptide, B-natriuretic peptide and procalcitonin are being studied to assess whether they serve as surrogate markers of diagnosis and mortality of CAP [13].
One of the main causes of mortality in CAP is sepsis, hence the need arose to identify patients who are already in sepsis as they require supervised treatment in wards or in critical care units. Also, if a patient is not in sepsis, then such patients can be treated at home if the treating physician finds no other clinical instability.
Procalcitonin, a 116- amino acid precursor of the hormone calcitonin is a biomarker which correlates to severity of bacterial infection [14–16]. PCT is markedly raised in sepsis upto 1000 times while it is low in viral infections [17,18]. Hence considerable interest has been shown in its usefulness as a biomarker for sepsis, to assess severity and prognosis of patients in sepsis [19,20].
Aims
This study was aimed to determine the ability of baseline serum procalcitonin level at predicting mortality in Community Acquired Bacterial Pneumonia (CABP) compared to PSI, CURB-65 and CRB-65. The secondary aim was to assess whether the combination of PCT to each score improves the ability to predict 30 day pneumonia specific mortality. We hypothesized that serum procalcitonin will aid in risk assessment in patients with CABP.
Materials and Methods
This was a prospective study conducted in the Pulmonary medicine wards of Goa Medical College and Hospital, Goa, India. After obtaining informed consent 55 patients admitted with CABP were enrolled from January 2011 till July 2012.
The inclusion criteria were males and females ≥ 15 years of age and CABP definition adapted from Bartlett et al., [21]; radiographically confirmed pneumonia (new or progressive infiltrates on CXR or CT scan consistent with bacterial pneumonia), acute illness (≤ 7 days duration) with at least three of the following clinical signs or symptoms consistent with a lower respiratory tract infection – new or increased cough, purulent sputum or change in sputum character, auscultatory findings consistent with pneumonia (e.g. rales, egophony, findings of consolidation), dsypnoea, tachypnoea or hypoxemia, fever greater than 380C oral or hypothermia (<350C), white blood cell count greater than 10,000 cells/mm3 or less than 4,500 cells/mm3, greater than 15% immature neutrophils (bands) irrespective of WBC count. We excluded patients with HIV seropositivity, on immunosuppressive drug therapy, pulmonary tuberculosis, pulmonary infarction, congestive cardiac failure, health-care associated pneumonia, aspiration pneumonia and patients with prior antibiotic therapy for the current pneumonia episode.
On admission patients demographic data was collected and laboratory workup for pneumonia was done including sputum sample for Gram stain and culture and blood for culture within one hour of admission. Baseline severity assessment scores were calculated (PSI, CURB-65, CRB-65) and serum procalcitonin was measured by semi-quantitative assay PCT- Q (BRAHMS Germany). This test was conducted by staff blinded to the clinical details of the patients. Patient was considered having severe pneumonia if, PCT level was ≥ 2 ng/ml, CURB- 65, CRB- 65 score of ≥3 and PSI score of IV and V. Patients were treated as per hospital’s standard protocol. We followed up on discharged patients for 30 day mortality, the traditional end point for clinical prediction rule in CAP. The study was approved by the Institutional Ethics Committee.
Measurement of Procalcitonin
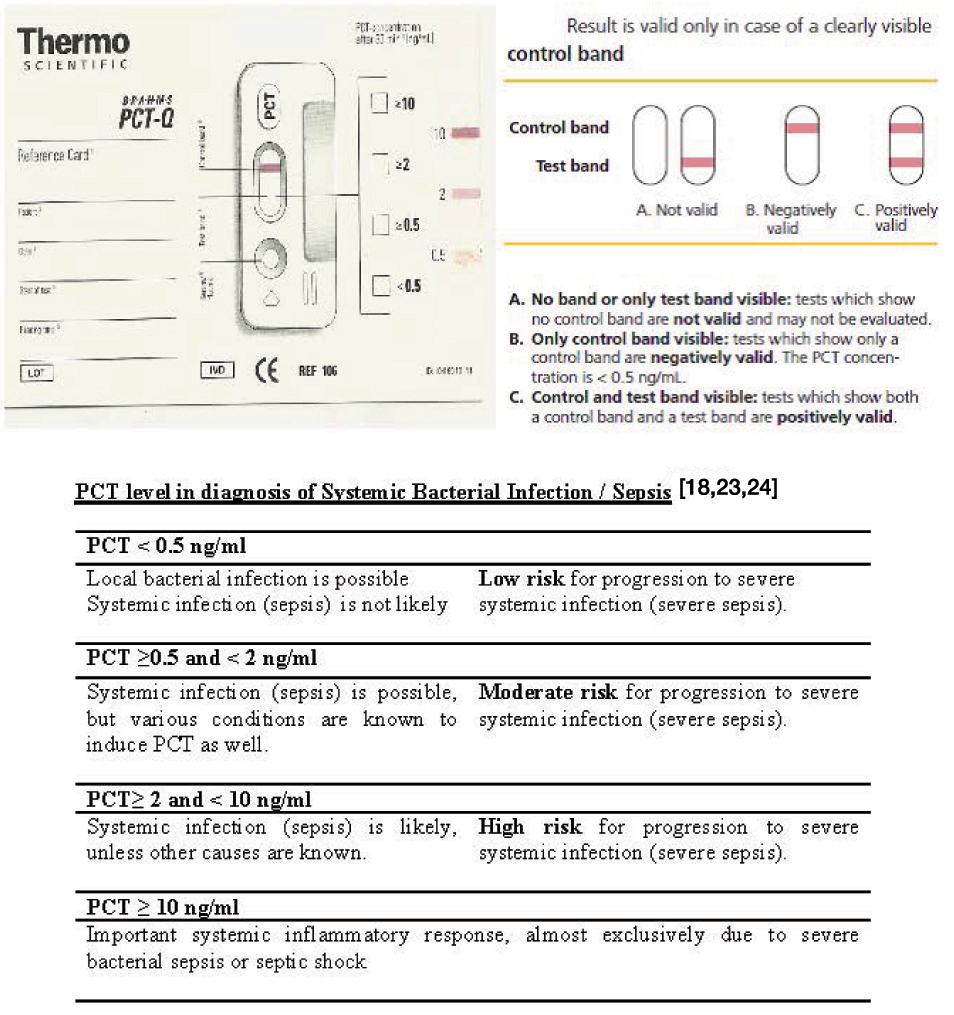
Thermo Fischer Scientific, PCT Q test kit is simple and easy to use. It relies on immunochromatographic principle. When patient’s serum is applied to the test kit, the semi- quantitative measurement results are obtained in half an hour. The result is measured by the intensity of the colour band which is compared to the bands on the reference card. Based on the colour, PCT level can be quantified broadly into 4 bands: < 0.5 ng/ml, ≥0.5 – 1.99 ng/ml, ≥2 – 9.99 ng/ml and ≥10 ng/ml. The validity of the test is ensured by appearance of a control band [22]. Serum or plasma procalcitonin concentrations of healthy persons measured with this assay are < 0.5 ng/ml, thus below the detection limit of the assay [Table/Fig-1].
PCT concentration levels [18,23,24]
Statistical Analysis
Continuous variables are expressed as mean ± SD or median and interquartile range (IQR) when normality assumptions of the distribution were not satisfied. Two group non parametric comparisons were calculated by the Mann Whitney U-test and t-test for normally distributed data. Frequency comparison was performed by Fisher’s exact test. Sensitivity, specificity, positive and negative predictive value of procalcitonin was compared to PSI, CURB-65 and CRB-65. The predictive ability of PCT combined with each of the three scores was also assessed. Receiver operating characteristic (ROC) curve and the area under the curve (AUC) was determined for each score. The outcome variable was 30 day mortality. All statistical tests were two tailed and a p-value of < 0.05 was considered statistically significant.
Results
Fifty five community acquired bacterial pneumonia patients were enrolled. The background characteristics of the study population are presented in the [Table/Fig-2]. In this study majority of patients were males (69.1%) compared to females (30.9%). The mean ±SD age of male patients was 44.2±16.1 years, and 46.4±17.3 years for female (p>0.05). The overall mortality rate was 10.9%. The mean±SD age of survived was 43.7±16.6 years, and 54.34±10.5 years for died patients and there was statistically no significant difference in mean age between the patients who died and survived.
Background characteristics
| n=55 | Number (%) | |
|---|
| Gender: |
| Male | 38 ( 69.1%) | |
| Female | 17 (30.9%) | |
| Outcome: |
| Survived | 49 (89.1%) | |
| Died | 6 (10.9%) | |
| Age (Years): | Mean±SD | |
| Male | 44.2±16.1 | t=0.65, df =49, p=0.64 |
| Female | 46.4±17.3 |
| Survived | 43.7±16.6 | t = 1.52, df=53, p= 0.13 |
| Died | 54.3±10.5 |
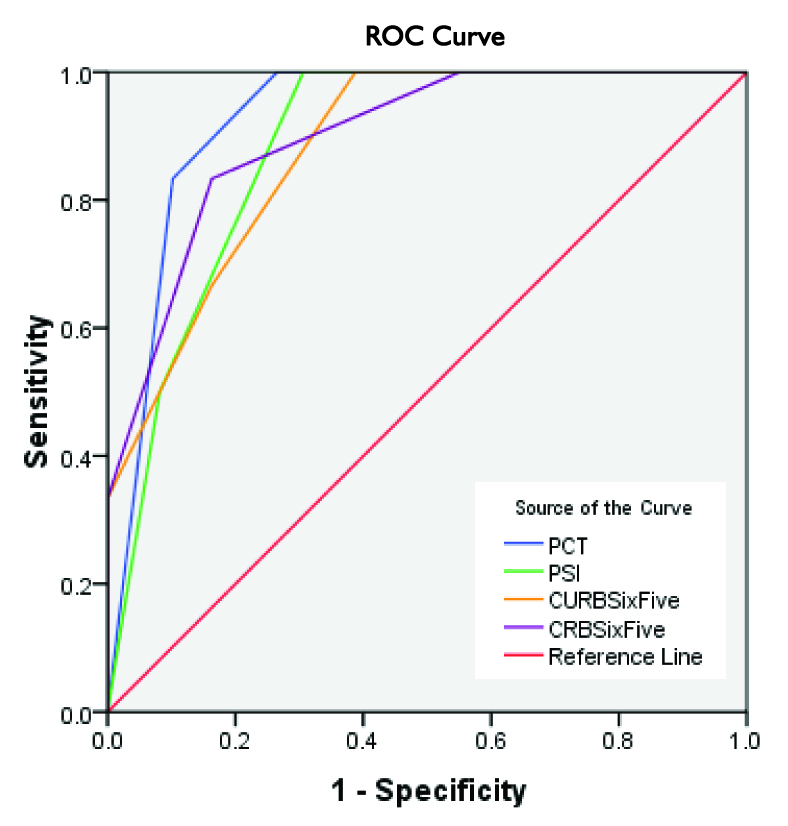
There were 20(36.4%) smokers. The median duration of hospital stay was 7.0 (6, 10) days. Number of deaths in CRB-65 risk class (0-4) was 0, 1, 3, 2 and 0 respectively, in CURB-65 risk class 0-5 was 0,0,2,2,2,0 respectively, PSI PORT risk class I-V was 0,0,0,3,3. [Table/Fig-3] shows scores between survivors and died. The Mann-Whitney Test clearly shows that median score was significantly higher for patients who died than those patients who survived for CRB-65, CURB-65, PCT and PSI. Number of deaths in PCT scores is shown in [Table/Fig-4]. PCT levels were high for increasing severity of CAP. Sensitivity, Specificity, Positive and Negative Predictive Value of all scores is shown in [Table/Fig-5]. [Table/Fig-6] shows the effect of PCT when combined with other scores. The accuracy of PCT, PSI, CURB-65 and CRB-65 to predict death at 30 days according to ROC is shown in [Table/Fig-7]. The ROC area under the curve (AUC) was highest for PCT.
Descriptive statistics of scores according to outcome in CRB-65, CURB-65, PCT and PSI
| Outcome | Min | Max | Mean±SD | Median | Inter Quartile Range | P-value |
|---|
| CRB-65 | Survived | 0 | 2 | 0.71±0.74 | 1 | 0-1 | Mann-Whitney U=29.5, p=0.001 |
| Died | 1 | 3 | 2.17±0.97 | 2 | 1.75-3 |
| CURB-65 | Survived | 0 | 3 | 1.37±0.97 | 1 | 1-2 | Mann-Whitney U=35.0, p=0.002 |
| Died | 2 | 4 | 3±0.89 | 3 | 2-4 |
| PCT | Survived | 1 | 4 | 1.94±1.01 | 2 | 1-3 | Mann-Whitney U=34.5, p=0.002 |
| Died | 3 | 4 | 3.83±0.41 | 4 | 3.75-4 |
| PSI | Survived | 1 | 5 | 2.61±1.35 | 3 | 1-4 | Mann-Whitney U=21.5, p=0.001 |
| Died | 4 | 5 | 4.5±0.55 | 4.5 | 4-5 |
Distribution of PCT Levels and Mortality
| PCT Level | Number of Patients n (%) | Number of Deaths |
|---|
| < 0.5 ng/ml | 21 (38.18) | 0 |
| 0.5 – 1.9 ng/ml | 15(27.27) | 0 |
| 2 – 9.9 ng/ml | 9 (16.36) | 1 |
| > 10 ng/ml | 10 (18.18) | 5 |
Sensitivity, Specificity, Positive Predictive Value and Negative Predictive Value of all scores for Mortality
| Severity Tool | Mortality |
|---|
| Sensitivity | Specificity | PPV | NPV |
|---|
| PCT ≥ 2 ng/ml | 100 | 73.47 | 31.58 | 100 |
| PSI IV + V | 100 | 69.39 | 28.57 | 100 |
| CURB-65 ≥ 3 | 66.67 | 83.67 | 33.33 | 95.35 |
| CRB-65 ≥ 3 | 33.33 | 100 | 100 | 92.45 |
Sensitivity, Specificity, Positive Predictive Value and Negative Predictive Value of Procalcitonin combined with each of the three scores
| Severity Tool | Mortality |
|---|
| Sensitivity | Specificity | PPV | NPV |
|---|
| PCT ≥ 2 ng/ml + PSI(IV+V) | 100 | 59.18 | 23.07 | 100 |
| PCT ≥ 2 ng/ml + CURB-65 ≥ 3 | 100 | 69.38 | 28.57 | 100 |
| PCT ≥ 2 ng/ml + CRB-65 ≥ 3 | 100 | 73.47 | 31.58 | 100 |
Receiver Operating Characteristic Curve, comparing PCT, PSI, CURB-65 and CRB-65 to predict mortality
 |
|---|
| Area Under the Curve |
|---|
| Test Result Variable(s) | Area | Std. Errora | Asymptotic Sig.b | Asymptotic 95% Confidence Interval |
|---|
| Lower Bound | Upper Bound |
|---|
| PCT | .927 | .038 | .001 | .853 | 1.001 |
| PSI | .883 | .051 | .002 | .783 | .982 |
| CURB-65 | .881 | .059 | .002 | .765 | .997 |
| CRB-65 | .900 | .060 | .002 | .782 | 1.018 |
Discussion
This study showed that the semi-quantitative PCT test is helpful in predicting 30 day mortality in adult patients with Community Acquired Bacterial Pneumonia. The sensitivity and specificity of procalcitonin of ≥ 2ng/ml to predict 30 day mortality was 100% and 73.47% respectively, the negative predictive value was 100 and the area under receiver operating characteristics curve was 0.92 (0.85 - 1.0). Kasamatsu Yu et al., in his study using similar semi-quantitative test with a cut off of ≥ 0.5ng/ml reported AUC for predicting 30 day mortality with PCT as 0.80 (0.70 – 0.90) [25]. A study by Kim et al., showed that the odds ratio for severe pneumonia showed a seven to eight fold increase for PSI and CURB-65 at PCT level of ≥ 2.0 ng/ml and the odds ratio for mortality had seven fold increase with PCT > 10ng/ml [26].
Huang DT, Menendez R and Kruger S in their studies reported that procalcitonin levels at admission predict outcome of pneumonia and identify patients at low risk of death [27–29]. We also found that if the existing risk scores predicted high risk for mortality and procalcitonin level assessed the patient as low risk, than these patients had a favourable outcome. Kruger et al., reported that readily measurable biomarkers that reflect the severity of CAP and outcome could be helpful as additional prognostic tools. He reported that a PCT threshold of ≤ 0.228 ng/ml was able to predict survivors despite an increased CRB-65 score [29].
Our study showed that PCT has similar prognostic accuracy as PSI to predict mortality, ([Table/Fig-5]). Man et al., [30] showed that PSI performs consistently as a predictor of mortality in CAP with AUC ranging from 0.74 to 0.83 while in our study the AUC for PSI ranged from 0.78 to 0.98. Similar observations were also made by Buising et al., who reported that the AUC for PSI ranged from 0.76 to 0.88 [31]
The British Thoracic Society introduced CURB-65 in 2003, having good discriminatory value and AUC ranging from 0.73 - 0.83. In our study the AUC for CURB-65 ranged from 0.76 to 0.99 while Buising et al., reported AUC from 0.76 – 0.88 and Aujesky reported AUC as 0.82 [32]. CRB-65 score AUC in our study ranged from 0.78 - 1.0. CRB-65 is a simple test and the CAPNETZ study showed that it provides comparable prediction of death in CAP [33].
Pneumonia severity assessment helps treating physicians identify which patients are at risk of dying. For this a tool with high sensitivity and a good negative predictive value is needed. Patients who are identified as not severe have a less likelihood of dying. When PCT value was added to PSI, CURB-65 and CRB-65, it improved the sensitivity and negative predictive value of all scores to 100% (See [Table/Fig-6]). Kruger et al., reported that a combined use of PCT and CRB-65 score optimizes the prognostic accuracy [29]. Hence, if we use the simple CRB-65 score along with procalcitonin, we will be able to predict mortality in CABP without using cumbersome methods.
Most studies comparing the predictive ability of PCT with pneumonia severity scores used the quantitative assay. This test requires costly equipment and regular calibration, which limits the utility of PCT measurements in resource limited settings. In our study we used the semi-quantitative assay PCT- Q which does not require any special apparatus or calibration and can give quick results. The validity of semi - quantitative test compared to quantitative analysis was demonstrated to be similar to support acute diagnostic decisions by Meisner et al., [22]. Kasamatsu et al., have studied the predictive ability of baseline serum PCT for mortality using the semi- quantitative kits and compared it to the A DROP, CURB-65 and PSI scores. They found that baseline serum PCT levels were positively correlated with the PSI and the CURB-65 [25]. They concluded that the baseline serum PCT level is useful for predicting 30 day mortality in adult patients with CAP which is similar to our findings.
Conclusion
The present study demonstrated that PCT level risk class at admission is an excellent test to predict 30 day mortality in community acquired bacterial pneumonia. PCT has a similar area under curve as CRB-65, an easy to use score for clinicians in busy ER. We emphasise that PCT should not be used in isolation but along with clinical judgement and a simple, easy to use CRB-65 score. PCT individually is a good screening test for 30 day mortality and when combined with any score, it improves the sensitivity and negative predictive value for mortality.
Abbreviations
PCT Procalcitonin
PORT PSI Pneumonia Outcome Research Team Pneumonia Severity Score
CURB-65 Confusion, Urea, Respiratory Rate, Blood Pressure, age ≥ 65 years
CRB-65 Confusion, Respiratory Rate, Blood Pressure,age ≥ 65 years
CABP Community Acquired Bacterial Pneumonia
NPV Negative Predictive Value
PPV Positive Predictive Value
IDSA Infectious Disease Society of America
[1]. Lutfyya MN, Henley E, Chang LF, Reyburn SW, Diagnosis and treatment of community-acquired pneumoniaAm Fam Physician 2006 73:442-50. [Google Scholar]
[2]. World Health Organization. Disease and injury country estimates. Accessed from WHO website on 29th Oct, 2014 [Google Scholar]
[3]. Fine TM Jr, Marrie TJ, Burden of community- acquired pneumonia in North American adultsPostgrad Med 2010 122(2):130-41. [Google Scholar]
[4]. Renaud B, Coma E, Labarere J, Hayon J, Roy PM, Boureaux H, Pneumocom Study Investigators. Routine use of the Pneumonia Severity Index for guiding the site-of-treatment decision of patients with pneumonia in the emergency department: a multicenter, prospective, observational, controlled cohort studyClin Infect Dis 2007 44(1):41-49. [Google Scholar]
[5]. Macfarlane JT, Boldy D, 2004 Update of BTS pneumonia guidelines: What’s new?Thorax 2004 59:364-66. [Google Scholar]
[6]. Fine MJ, Auble TE, Yealy DM, Hanusa BH, Weissfeld LA, Singer DE, A prediction rule to identify low-risk patients with community-acquired pneumoniaN Engl JMed 1997 336(4):243-50. [Google Scholar]
[7]. Lim WS, van der Eerden MM, Laing R, Boersma WG, Karalus N, Town G, Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation studyThorax 2003 58(5):377-82. [Google Scholar]
[8]. Collini P, Beadsworth M, Anson J, Neal T, Burnham P, Deegan P, Community-acquired pneumonia: doctors do not follow national guidelinesPostgrad Med J 2007 83:552-55. [Google Scholar]
[9]. Sharma BB, Singh V, Indian pneumonia guidelinesLung India 2012 29:307-08. [Google Scholar]
[10]. Lee RW, Lindstrom ST, A teaching hospital’s experience applying the Pneumonia Severity Index and antibiotics guidelines in the management of community-acquired pneumoniaRespirology 2007 12(5):754-58. [Google Scholar]
[11]. Aujesky D, McCausland JB, Whittle J, Obrosky DS, Yealy DM, Fine MJ, Reasons why emergency department providers do not rely on the pneumonia severity index to determine the initial site of treatment for patients with pneumoniaClin Infect Dis 2009 49(10):e100-18. [Google Scholar]
[12]. Singanayagam A, Chalmers JD, Hill AT, Severity assessment in community-acquired pneumonia: a reviewQ J Med 2009 102:379-88. [Google Scholar]
[13]. Huang DT, Angus DC, Kellum JA, Pugh NA, Weissfeld LA, Struck J, Midregional proadrenomedullin as a prognostic tool in community-acquired pneumoniaChest 2009 136(3):823-31. [Google Scholar]
[14]. Becker KL, Nylén ES, White JC, Müller B, Snider RH Jr, Clinical review 167: Procalcitonin and the calcitonin gene family of peptides in inflammation, infection, and sepsis: a journey from calcitonin back to its precursorsJ Clin Endocrinol Metab 2004 89(4):1512-25. [Google Scholar]
[15]. Müller B, Becker KL, Schächinger H, Rickenbacher PR, Huber PR, Zimmerli W, Calcitonin precursors are reliable markers of sepsis in a medical intensive care unitCrit Care Med 2000 28(4):977-83. [Google Scholar]
[16]. Masiá M, Gutiérrez F, Shum C, Padilla S, Navarro JC, Flores E, Usefulness of procalcitonin levels in community-acquired pneumonia according to the patients outcome research team pneumonia severity indexChest 2005 128(4):2223-29. [Google Scholar]
[17]. Simon L, Gauvin F, Amre DK, Saint–Louis P, Lacroix J, Serum procalcitonin and C-reactive protein levels as markers of bacterial infections: a systematic review and meta- analysisClin Infect Dis 2004 39(2):206-17. [Google Scholar]
[18]. Christ-Crain M, Muller B, Procalcitonin in bacterial infections—hype, hope, more or less?Swiss Med Wkly 2005 135(31-32):451-60. [Google Scholar]
[19]. Assicot M, Gendrel D, Carsin H, Raymond J, Guilbaud J, Bohuon C, High serum procalcitonin concentrations in patients with sepsis and infectionLancet 1993 341(8844):515-18. [Google Scholar]
[20]. Aikawa N, Fujishima S, Endo S, Sekine I, Kogawa K, Yamamoto Y, Multicenter prospective study of procalcitonin as an indicator of sepsisJ Infect Chemother 2005 11(3):152-59. [Google Scholar]
[21]. Bartlett JG, Dowell SF, Mandell LA, File Jr TM, Musher DM, Fine MJ, Practice guidelines for the management of community-acquired pneumonia in adults. Infectious Diseases Society of AmericaClin Infect Dis 2000 31(2):347-82. [Google Scholar]
[22]. Meisner M, Brunkhorst FM, Reith HB, Schmidt J, Lestin HG, Reinhart K, Clinical experiences with a new semi-quantitative solid phase immunoassay for rapid measurement of procalcitoninClin Chem Lab Med 2000 38(10):989-95. [Google Scholar]
[23]. Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. 1992Chest 2009 136(5 Suppl):e28 [Google Scholar]
[24]. Brunkhorst FM, Wegscheider K, Forycki ZF, Brunkhorst R, Procalcitonin for early diagnosis and differentiation of SIRS, sepsis, severe sepsis and septic shockIntensive Care Med 2000 26(Suppl 2):S148-52. [Google Scholar]
[25]. Kasamatsu Y, Yamaguchi T, Kawaguchi T, Tanaka N, Oka H, Nakamura T, Usefulness of a semi-quantitative procalcitonin test and the A-DROP Japanese prognostic scale for predicting mortality among adults hospitalized with community-acquired pneumoniaRespirology 2012 17(2):330-36. [Google Scholar]
[26]. Kim JH, Seo JW, Mok JH, Kim MH, Cho WH, Lee K, Usefulness of plasma procalcitonin to predict severity in elderly patients with community-acquired pneumoniaTuberc Respir Dis (Seoul) 2013 74(5):207-14. [Google Scholar]
[27]. Huang DT, Weissfeld LA, Kellum JA, Yealy DM, Kong L, Martino M, GenIMS Investigators. Risk prediction with procalcitonin and clinical rules in community-acquired pneumoniaAnn Emerg Med 2008 52(1):48-58. [Google Scholar]
[28]. Menéndez R, Martínez R, Reyes S, Mensa J, Filella X, Marcos MA, Biomarkers improve mortality prediction by prognostic scales in community-acquired pneumoniaThorax 2009 64(7):587-91. [Google Scholar]
[29]. Krüger S, Ewig S, Marre R, Papassotiriou J, Richter K, von Baum H, CAPNETZ Study Group. Procalcitonin predicts patients at low risk of death from community-acquired pneumonia across all CRB-65 classesEur Respir J 2008 31(2):349-55. [Google Scholar]
[30]. Man SY, Lee N, Ip M, Antonio GE, Chau SS, Mak P, Prospective comparison of three predictive rules for assessing severity of community-acquired pneumonia in Hong KongThorax 2007 62(4):348-53. [Google Scholar]
[31]. Buising KL, Thursky KA, Black JF, MacGregor L, Street AC, Kennedy MP, A prospective comparison of severity scores for identifying patients with severe community acquired pneumonia: reconsidering what is meant by severe pneumoniaThorax 2006 61:419-24. [Google Scholar]
[32]. Aujesky D, Auble TE, Yealy DM, Stone RA, Obrosky DS, Meehan TP, Prospective comparison of three validated prediction rules for prognosis in community-acquired pneumoniaAm J Med 2005 118(4):384-92. [Google Scholar]
[33]. Bauer TT, Ewig S, Marre R, Suttorp N, Welte T, the CAPNETZ study group. CRB-65 predicts death from community-acquired pneumoniaJ Intern Med 2006 260(1):93-101. [Google Scholar]