Introduction
Angiogenesis is a complex process in which there is growth of new blood vessels from the pre-existing ones and is an essential phenomenon for the growth and survival of solid neoplasms [1].Tumour angiogenesis is the proliferation of blood vessels penetrating the cancerous growth for the supply of nutrients and oxygen [2]. Angiogenesis is a requisite not only for continued tumour growth, but also for metastasis [3]. Because adequate vascular response is critical for the initial development as well as the continued growth of solid tumours, much attention is focused on the use of angiogenesis inhibitors as an adjunct to other forms of therapy for preventing development of malignant neoplasms [4].
Text
Physiological Angiogenesis Mechanism
Vasculogenesis is a process in which blood vessels are assembled during embryonic development and further transformation of vascular net which proceed during angiogenesis, it is a complex multistep process where new vessels are formed from the pre-existing ones [3]. Steps in angiogenesis are shown in [Table/Fig-1].
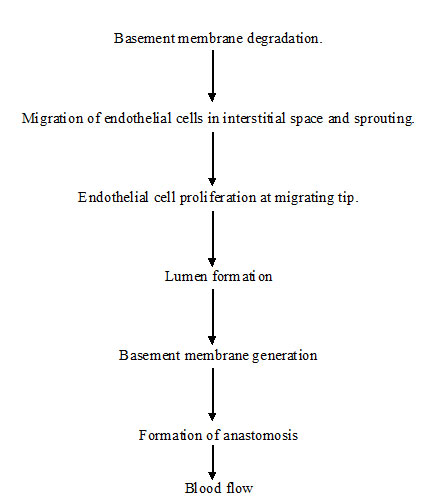
Tumour angiogenesis
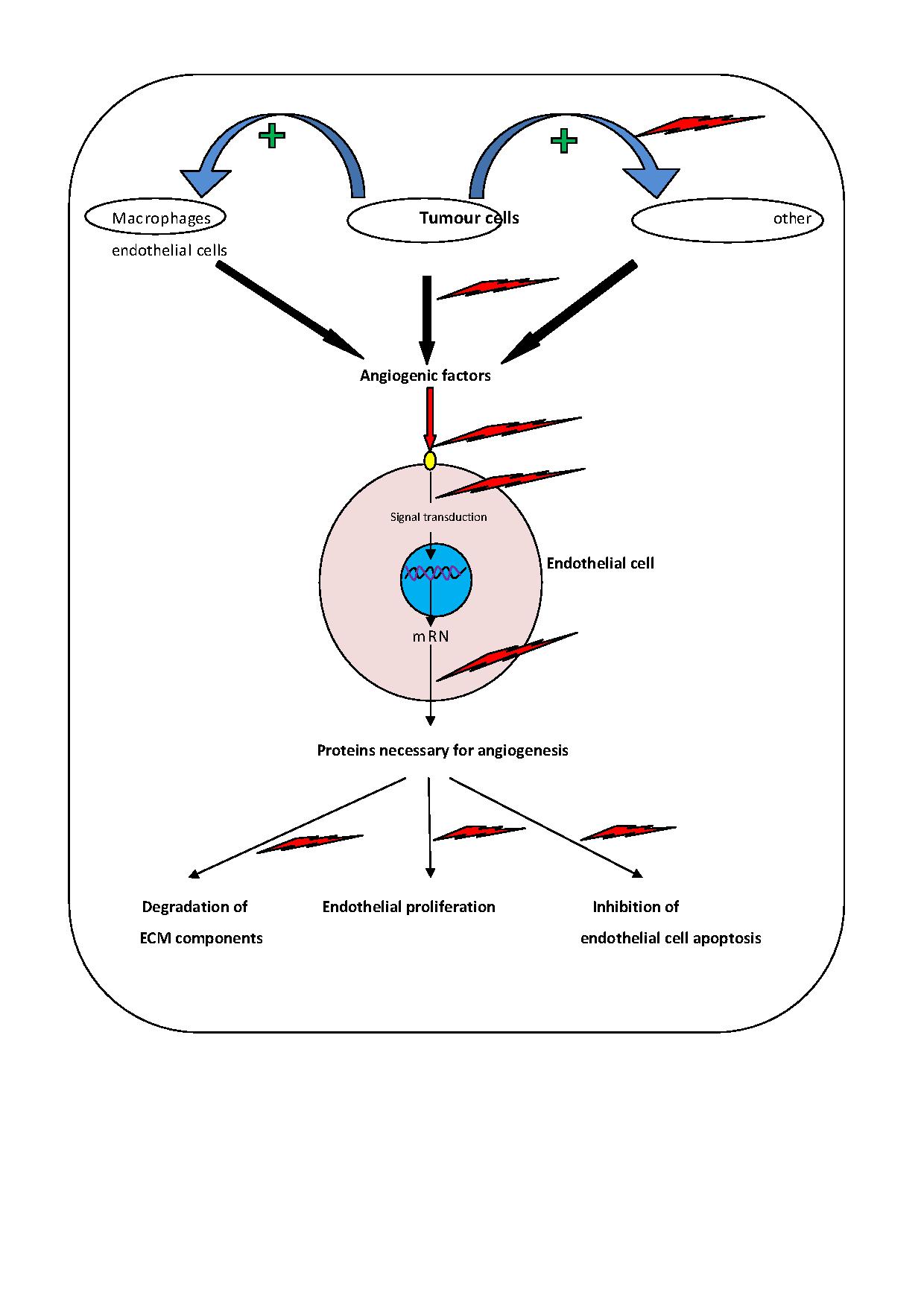
Angiogenesis is the process of new vessel formation and hallmark of tumour progression [5]. Folkman and colleagues demonstrated that solid tumours cannot grow larger than 2-3 mm diameter without inducing their own blood supply [6]. Tumour angiogenesis starts with the release of molecules by tumour cells that send signals to the surrounding normal host tissue, activates certain genes to make protein that encourage growth of new blood vessels [2].
Angiogenic Switch Mechanism
According to the experimental and clinical data most human tumours do not induce angiogenesis and exist in situ, without blood supply for months to years, when some cells within small tumour change to an angiogenic phenotype by a phenomenon known as angiogenic switch. The molecular basis of this mechanism may be increased production of angiogenic factors or loss of angiogenesis inhibitors [Table/Fig-2,3] [3]. Thus, the switch to an angiogenic phenotype is regulated by a change in the equilibrium between positive and negative regulators of angiogenesis [7].
Normal angiogenic mechanism
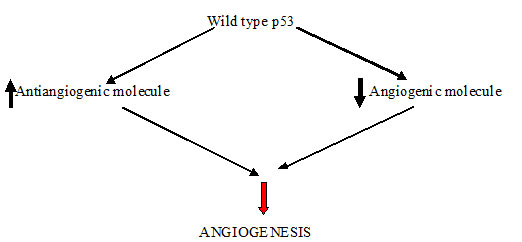
Angiogenic switch (Tumour induced)
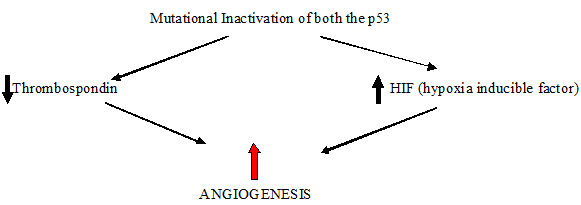
Factors in Tumour Angiogenesis
Most blood vessels in an adult organism remain quiescent but have the capability to divide in response to stimulus and result in angiogenic process. The molecules that are the positive regulators of angiogenesis are:
Vascular endothelial growth factor (VEGF)
Platelet Derived Growth Factor (PDGF)
Fibroblast growth factor (FGF)
Epidermal growth factor (EGF)
Transforming growth factor (TGF)
Matrix metalloproteinase’s (MMP’s)
TNF (Tumour necrosis factor)
Angiopoietins [8].
A-VEGF (Vascular endothelial growth factor): VEGF also known as vascular permeability factor (VPF) is a heparin binding protein and its level is increased in various tumours [9].
Actions of VEGF
Powerful inducer of angiogenesis [10].
Stimulates growth and proliferation of endothelial cells [8].
Act as survival factor for endothelial cells [11].
Prevent the apoptosis of endothelial cells [12].
Regulates the vascular permeability [13].
Structure of VEGF
VEGF is a secreted protein which promotes angiogenesis in tumours, chronic inflammation and healing of wounds. Gene encoding the human VEGF consists of 8 exons and 7 introns [3,14]. Alternative splicing of VEGF gene results in 4 different isoforms VEGF 121, VEGF165, VEGF 189, VEGF 206 [14] and less frequent spliced isoforms are VEGF 145 and VEGF 183 [8]. First 26 AAs in VEGF constitute signalling peptide [14]. The most frequent isoform is VEGF 165 which is a homodimer and has a molecular mass of 45 kD [15].
Hypoxia is one of the important factors inducing VEGF expression.[16] Other inducing agents are EGF, TGF α & β, IGF-1, FGF and PDGF [17]. Hypoxia induced transcription of VEGF mRNA is mediated by the hypoxia inducible factor -1 (HIF-1), the binding site of which is located in the VEGF promoter region [18].
VEGF Family & Receptors
Consist of closely related factors that are VEGF A (VEGF), VEGF B, VEGF C, VEGF D, VEGF E and placental growth factor (PIGF) [19]. VEGF family members signal through three tyrosine kinase receptors VEGF R1, VEGF R2, VEGF R3 [3]. Like all RTK, VEGF receptors are transmembranous proteins with a single transmembrane domain. Extracellular region are formed by seven immunoglobulin - like domain. Intracellular region exhibits tyrosine kinase activity and separated to two fragments (TK1and TK2) by an inter kinase insert [20]. VEGF R2 is located in endothelial cells and is the main receptor for the vasculogenic and angiogenic effects of VEGF.
B- Platelet Derived Growth Factor (PDGF): Family of PDGF ligand is composed of 4 structurally related soluble peptides in the form of 5 different homodimers & heterodimers and involved in vessel maturation and recruitment of pericytes [19].
C- Fibroblast Growth Factor (FGF): It is comprised of 23 different proteins and classified in to 6 different groups. These ligands are among the earliest angiogenic factors and involved in promoting cell proliferation, migration and differentiation of vascular ECs [2].
D-Epidermal Growth Factor (EGF): It is comprised of 11 members and 4 EGF receptors. Activation of EGFR pathway results in up regulation of proangiogenic factors such as VEGF and thus viewed as indirect regulator of angiogenesis [5].
E- Transforming Growth Factor-β (TGF β): TGF β is produced by nearly every cell type and participates in angiogenesis, embryonic development & wound healing and has potent growth inhibition properties. It has both pro and anti angiogenic properties, depending on its levels. Low levels promote angiogenesis by up regulating angiogenic factors & proteases and high levels inhibit ECs growth and proliferation by preventing phosphorylation of pRB and thereby arresting endothelial cells at late G1 phase [2].
F-Matrix Metalloproteinases (MMPs): MMPs induce tumour angiogenesis by degrading ECM and releasing angiogenic mitogens that are stored in the matrix. MMP-9 and MMP-2 proteolytically cleave and activate latent TGF-β and promote tumour angiogenesis [2].
G-TNF (Tumour necrosis factor): TNF is a cytokine released from macrophage, mast cells and T-lymphocytes. It acts as a macrophage activating factor and activates these cells to secrete angiogenic factors.
H-Angiopoietins: Angiopoietin 1 and 2 can act both as proangiogenic and anti angiogenic because of their respective agonist and antagonist signal through Tie receptors. Ang 1 stimulates Tie 2 while Ang 2 does not activate receptor and act as competitive inhibitor of Ang 1.
Angiogenesis Inhibitors
Because an adequate vascular response is essential for the initial development and the continued growth of the solid tumours so now a day’s anti angiogenic therapy is an attractive modality for preventing the development of malignant neoplasm [21]. The angiogenic inhibitors can be either endogenous (present within the body) or synthetic (drugs).
Different mechanisms of antiangiogenesis are based on the step of angiogenic cascade that is inhibited which can be as under [Table/Fig-4].
Angiogenesis cascade and steps blocked by angiogenesis inhibitors
Prevent or decrease the secretion of angiogenic factors by tumour cells e.g.-interferon [22].
Increase the secretion of antiangiogenic factors e.g.: retinoids [1]
Prevent the activation of macrophages and other endothelial cells by the tumour cells [23].
Targeting the actions of VEGF [24].
Inhibition of proteases that is essential for penetration of basement membrane and degradation of surrounding ECM to create space in which endothelial cells can proliferate and form new vessels [24]. E.g.- Merimastat, Neovastat
Induce the EC apoptosis [24].
Inhibition of EC survival [24].
To make the endothelial cells refractory to angiogenic stimulus [1]. E.g.-Thalidomide, endostatin, squalamine,TNP-470
The various Endogenous Angiogenesis inhibitors are as follows:
Interferon
Interleukins
TIMP
Angiostatin
Endostatin
A. Interferon
Interferon is the members of secreted glycoproteins which directly or indirectly inhibit the tumour angiogenesis and growth [25]. Administration of optimal dose of IFN α/β decrease the expression of β - FGF m RNA and protein, microvessel density and also induces the apoptosis of endothelial cells [25]. IFN γ induces its antiangiogenic effects through the secretion of IFN γ – inducible protein 10 (IP-10) and monokine [26].
The structure of interleukins (ILs) determines its function to play a role in either promoting or inhibiting angiogenesis [Table/Fig-5].
Role of Interleukins in angiogenesis
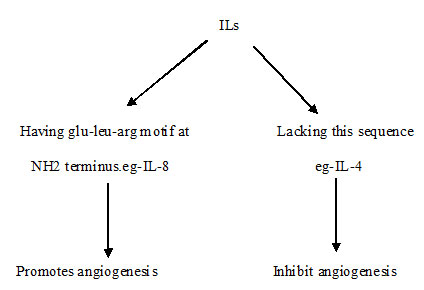
IL1a is a cytokine secreted by activated macrophage induces angiogenesis through the increased expression of angiogenic factors [28]. IL 12 suppresses the expression of VEGF mRNA, promotes the apoptosis and inhibits proliferation rate in human tumours and reduce tumour vessel density [29,30]. IL-10 down regulate the synthesis of VEGF, IL-1β, TNFα, IL-6, MMP 9 in tumour associated macrophages [31].
C. Timp
Degradation of basement membrane and remodelling of ECM is required to create a space in which endothelial cells can migrate and proliferate [32]. In the process of remodelling of ECM, matrix metalloproteinases (MMPs) have the central role and tissue inhibitors of matrix metalloproteinase’s (TIMP) inhibit the neovascularisation by inhibiting the breakdown of surrounding matrix. The migration of ECs through gelatine is inhibited by TIMP-1.TIMP-2 inhibits the β FGF induced EC proliferation [33]. Because of the multiple effects of TIMPs, MMPs are the attractive targets for tumour therapy.
D. Angiostatin
It is a 38 KDa internal fragment of plasminogen and its antiangiogenic effect is due to down regulation of VEGF expression within tumours [34]. Binding of angiostatin to plasma membrane localized ATP synthatase suppress the endothelial –surface ATP metabolism and thus down regulate the EC (endothelial cell) proliferation and migration [35]. According to many studies angiostatin treatment significantly increases the apoptosis of EC [36].
E. Endostatin
It is a 20 KDa fragment of type XVIII collagen and inhibits ECs proliferation, angiogenesis and tumour growth.
Mode of action of Endostatin
Inhibits the binding of VEGF to ECs.
Directly binds to receptors but not to VEGF.
Blocks the VEGF induced tyrosine phosphorylation.
Suppresses the VEGF induced downstream events of KDR/Flk-1 signalling which are involved in the mitogenic activities of VEGF [37].
Drugs That Target VEGF
Mode of action of VEGF includes ligand binding to the extracellular domain of transmembrane tyrosine kinase receptor which induces the autophosphoryation of an intracellular kinase domain and then subsequent downstream signalling by the kinases.
Drugs that inhibit the action of VEGF can act by any of the following mechanisms that can disrupt the above sequence as follows [Table/Fig-6]:
Drugs targeting Actions of VEGF
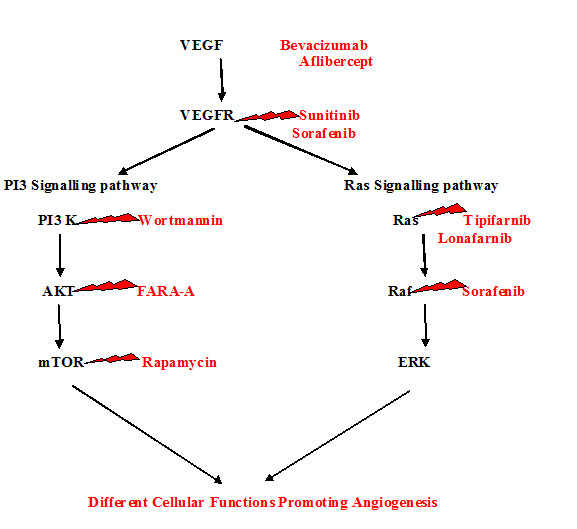
Anti VEGF mAb - Which directly neutralises the VEGF proteins and inhibits the biological actions of VEGF [38]? E.g. -Bevacizumab, Aflibercept.
Drugs which are soluble VEGF receptors that specifically bind to VEGF and block the binding of VEGF with actual receptors [39].
Drugs that is able to bind to VEGF receptors and thus act as inhibitors of VEGF receptors. E.g. -Sunitinib, Sorafenib
Inhibitors of VEGF signal transduction pathway by blocking autophosphorylation of VEGF receptors. e.g. -LY294002, Wortmannin, FARA-A, Rapamycin, Temsirolimus, Everolimus, Tipifarnib, Lonafarnib.
Drugs with a specific nucleotide sequence which is VEGF antisense and bind to VEGF mRNA and then interferes with the translation process and blocks the VEGF protein formation [40].
1. Drugs that Target Growth Factors
BEVACIZUMAB: It is a humanized monoclonal antibody, binds to VEGF A and prevents it from binding to receptors thus blocks the further signal transduction. Currently tested in various clinical trials for a variety of different tumours and in 2009 was approved for colorectal cancer, breast cancer and RCC [41].
AFLIBERCEPT-(VEGF-Trap, AVE 0005): It is a soluble fusion protein of extracellular domains of VEGFR1 and R2 and Fc portions of human IgG. Binds to VEGF A & PIGF and renders the ligands unavailable to bind and activate receptors [42].
2. Drugs That Target Receptor Trasncriptase Kinase (RTK)
SUNITINIB (SU 11248): Orally available tyrosine kinase inhibitor with activity against VEGFR, PDGFR, Flt-3, C-kit& RET, CSF 1R. Studies reported with sunitinib showed no significant antitumor activity in monotherapy. This drug has got FDA approval in 2006 for gastrointestinal stromal tumours and advanced metastatic RCC [43].
SORAFENIB (BAY 43-9006): Orally available inhibitor of intracellular Raf kinase and targets MAPK, Raf/MEK/ERK signalling pathways. It also inhibits VEGF R, PDGFR-β and C-kit [44].
3. Drugs that Target PI3K/ AKT/ m -TOR Pathway
This signalling pathway is responsible for many processes including angiogenesis, proliferation, and survival and is initiated by RTK activation [45].
LY294002 and wortmannin are inhibitors of PI3K pathway but demonstrated unacceptable level of toxicity in animals [46].
Temsirolimus (CCI-779) and Everolimus (RAD001) are inhibitors of mTOR. Clinical trials demonstrated improved survival in advanced RCC leading to FDA approval [47].
4. Drugs that Target MAPK - Farnesyltransferase Rho and Ras
MAPK signalling can lead to increased angiogenesis, thus making it a logical target for Antiangiogenic therapy [48]. The drugs that inhibit MAPK signalling are Tipifarnib (R115777) [49] Lonafarnib (SCH66336) [50].
5. Other drugs
Tnp-470 inhibits methionine aminopeptidase, prevents endothelial activation and arrest cell cycle [51].
Thalidomide inhibits TNF α synthesis leading immunomodulatory and anti – inflammatory effects, thus contributing to its Antiangiogenic effect [52].
Conclusion
Because fundamental requirement of tumour growth is vascular supply so antiangiogenic therapy for cancer is a highly effective strategy which represent a treatment, not cure and expected to be cytostatic, so particularly effective in combination with cytotoxic agents. One of the major challenges in designing of antiangiogenic therapy is to include each of the possible mechanisms by which tumours can induce new blood vessel growth.
[1]. Hasina R, Lingen MW, Angiogenesis and oral cancerJournal of Dental Education 2001 65(11):1282-90. [Google Scholar]
[2]. Gupta MK, Qin RY, Mechanism and its regulation of tumour induced angiogenesisWorld journal of gastroenterol 2003 9(6):1144-55. [Google Scholar]
[3]. Kumar V, Abbas AK, Fausto N, Robbins and Cotran Pathologic basis of disease 2004 7th edPhiladelphiaSaunders; Elsevier Inc [Google Scholar]
[4]. Hori A, Suppression of solid tumour growth by immune neutralising monoclonal antibody against human fibroblast growth factorCancer Res 1991 51:6180-84. [Google Scholar]
[5]. Carla C, Daris F, Cecilia B, Francesca B, Francesca C, Paolo F, Angiogenesis in head and neck cancer: A review of literatureJournal of oncology 2012 ID 358472 [Google Scholar]
[6]. Folkman J, Tumour Angiogenesis. In: Mendelsohn J, Howley PM, Israel MA, Liotta LA, edsThe Molecular Basis of Cancer 1995 PhiladelphiaWB Saunders:206-32. [Google Scholar]
[7]. Bussolino F, Mantovani A, Persico G, Molecular mechanism of blood vessel formationTrends Biochem Sci 1997 22:251-56. [Google Scholar]
[8]. Ferrara N, Davis Smyth T, The biology of vascular endothelial growth factorEndocr Rev 1997 18:4-25. [Google Scholar]
[9]. Kohn S, Nagy JA, Dvorak HF, Dvorak AM, Pathways of macromolecular tracer transport across venules and small veins. Structural basis for hyperpermeability of tumour blood vesselsLab Invest 1992 67:596-607. [Google Scholar]
[10]. Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N, Vascular endothelial growth factor is a secreted angiogenic mitogenScience 1989 246:1306-09. [Google Scholar]
[11]. Gerber HP, Dixit V, Ferrara N, Vascular endothelial growth factor induces expression of the antiapoptotic proteins Bcl-2 and A1 in vascular endothelial cellsJ Biol Chem 1998 273:13313-16. [Google Scholar]
[12]. Gerber HP, McMurtrey A, Kowalski J, Yan M, Keyt BA, Dixit V, Ferrara N, Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3 -kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activationJ biol. Chem 1998 273(46):30366-43. [Google Scholar]
[13]. Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF, Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluidScience 1983 219:983-85. [Google Scholar]
[14]. Houck KA, Ferrara N, Winer J, Cachianes G, Li B, Leung DW, The vascular endothelial growth factor family: identification of a fourth molecular species and characterization of alternative splicing of RNAMol Endocrinol 1991 5:1806-14. [Google Scholar]
[15]. Ferrara N, Henzel WJ, Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cellsBiochem Biophys Res Commun 1989 161:851-58. [Google Scholar]
[16]. Dor Y, Porat R, Keshet E, Vascular endothelial growth factor and vascular adjustments to perturbations in oxygen homeostasisAm J Physiol Cell Physiol 2001 280:C1367-74. [Google Scholar]
[17]. Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z, Vascular endothelial growth factor (VEGF) and its receptorsFASEB J 1999 13(1):9-22. [Google Scholar]
[18]. Mazure NM, Chen EY, Laderoute KR, Giaccia AJ, Induction of vascular endothelial growth factor by hypoxia is modulated by a phosphatidylinositol3-kinase/Akt signalling pathway in Ha-ras-transformed cells through a hypoxia inducible factor-1 transcriptional elementBlood 1997 90:3322-31. [Google Scholar]
[19]. Karamysheva AF, Mechanism of angiogenesisBiochemistry 2008 73:935-48. [Google Scholar]
[20]. Shibuya M, Yamaguchi S, Yamane A, Nucleotide sequence and expression of a novel human receptor-type tyrosine kinase gene (flt) closely related to the fms familyOncogene 1990 (5):519-24. [Google Scholar]
[21]. Kim DJ, Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour cell growth in vivoNature 1993 362:841-44. [Google Scholar]
[22]. Singh RK, Gutman M, Bucana CD, Sanches R, Liansa N, Fidler IJ, Interferons a down regulate the expression of basic fibroblast growth factor in human carcinomasProc Natl Acad Sci USA 1995 92:4562-66. [Google Scholar]
[23]. Vukanovic J, Issacs JT, Linomide inhibites angiogenesis, growth, metastasis and macrophage infiltration within rat prostatic cancersCancer Res 1995 55:1499-504. [Google Scholar]
[24]. Oehler MK, Bicknell R, The promise of anti-angiogenic cancer therapyBr j Cancer 2000 82:749-52. [Google Scholar]
[25]. Slaton JW, Perrotte P, Inoue K, Dinney CP, Fidler IJ, Interferon a mediated downregulation of angiogenesis related genes and therapy of bladder cancer are dependent on optimization of biological dose and scheduleClin Cancer Res 1999 5:2726-34. [Google Scholar]
[26]. Jonasch E, Haluska FG, Interferon in oncological practice: review of interferon biology, clinical applications and toxicitiesOncologist 2001 6:34-55. [Google Scholar]
[27]. Strieter RM, Polverini PJ, Kunkel SL, Arenberg DA, Burdick MD, Kasper J, The functional role of the ELR motif in CXC chemokines-mediated angiogenesisJ Biol Chem 1995 270:27348-57. [Google Scholar]
[28]. Torisu H, Ono M, Kiryu H, Furue M, Ohmoto Y, Nakayama J, Macrophage infiltration correlates with tumour stage and angiogenesis in human malignant melanomaInt J Cancer 2000 85:182-88. [Google Scholar]
[29]. Oshikawa K, Rakhmilevich AL, Shi F, Sondel PM, Yang N, Mahvi DM, Interleukin 12 gene transfer in to skin distant from the tumour site elicites antimetastatic effects equivalent to local gene transferHum Gene Ther 2010 12:149-60. [Google Scholar]
[30]. Duda DG, Sunamura M, Lozonschi L, Kodoma T, Egawa S, Matsumoto G, Direct in vitro evidence and in vivo analysis of the antiangiogenesis effects of interleukin -12Cancer Res 2000 60:1111-16. [Google Scholar]
[31]. Huang S, Ullrich SE, Bar-Eli M, Regulation of tumour growth and metastasis by interleukin-10: the melanoma experienceJ Interferon Cytokine Res 1999 19:697-703. [Google Scholar]
[32]. Fernandez HA, Kallenbach K, Seghezzi G, Grossy E, Colvin S, Schneider R, Inhibition of endothelial cell migration by gene transfer of tissue inhibitor of metalloproteinases-1J Surg Res 1999 82:156-62. [Google Scholar]
[33]. Murphy AN, Unsworth EJ, Stetler–Stevenson WG, Tissue inhibitor of metalloproteinases 2 inhibits bFGF induced human microvascular endothelial cell proliferationJ Cell Physiol 1993 157:351-58. [Google Scholar]
[34]. Hajitou A, Grignet C, Devy L, Berndt S, Blacher S, Deroanne CF, The antitumoural effect of endostatin and angiostatin is associated with a downregulation of vascular endothelial growth factor expression in tumour cellsFASEB J 2002 16:1802-04. [Google Scholar]
[35]. Moser TL, Stack MS, Asplin I, Enghild JJ, Hojrup P, Everitt L, Angiogenesis binds ATP synthase on the surface of human endothelial cellsProc Natl Acad Sci USA 1999 96:2811-16. [Google Scholar]
[36]. Griscelli F, Li H, Bennaceur-Griscelli A, Soria J, Opolon P, Soria C, Angiostatin gene transfer; inhibition of tumour growth in vivo by blockage of endothelial cell proliferation associated with a mitosis arrestProc Natl Acad Sci USA 1998 95:6367-72. [Google Scholar]
[37]. Kim YM, Hwang S, Kim YM, Pyun BJ, Kim TY, Lee ST, Endostatin blocks vascular endothelial growth factor mediated signalling via direct interaction with KDR/Flk-1J Biol Chem 2002 277:27872-79. [Google Scholar]
[38]. Zhang W, Ran S, Sambade M, Huang X, Thorpe PE, A monoclonal antibody that blocks VEGF binding to VEGF R2 inhibits vascular expression of Flk-1 and tumour growth in an orthotopic human breast cancer modelAngiogenesis 2002 5:35-44. [Google Scholar]
[39]. Witte L, Hicklin DJ, Zhu Z, Pytowski B, Kotanides H, Rockwell P, Bohlen P, Monoclonal antibodies targeting the VEGF receptor 2 as an antiangiogenic therapeutic strategyCancer Metastasis Rev 1998 17:155-61. [Google Scholar]
[40]. Im SA, Gomez Manzano C, Fueyo J, Liu TJ, Ke LD, Kim JS, Antiangiogenesis treatment for gliomas: transfer of antisense-vascular endothelial growth factor inhibits tumour growth in vivoCancer Res 1999 59:895-900. [Google Scholar]
[41]. Kim KJ, Li B, Winer J, Inhibition of vascular endothelial growth factor induced angiogenesis suppresses tumour growth in vivoNature 1993 362:841-44. [Google Scholar]
[42]. Holash J, Davis S, Papadopoulos N, VEGF Trap: a VEGF blocker with potent antitumour effectsProc Natl Acad Sci USA 2002 99:11393-98. [Google Scholar]
[43]. Gan HK, Seruga B, Knox JJ, Sunitinib in solid tumoursExpert Opin Investing Drugs 2009 18:821-34. [Google Scholar]
[44]. Wilhelm S M, Adnane L, Newell P, Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signallingMol Cancer Ther 2008 7:3129-40. [Google Scholar]
[45]. Engelman JA, Targetting PI3 signalling in cancer: opportunities, challenges and limitationsNat Rev cancer 2009 9:550-62. [Google Scholar]
[46]. Jiang BH, Jiang G, Zheng JZ, Phosphatdylinositol 3-kinase signalling controls levels of hypoxia inducible factor 1Cell growth Differ 2001 12:363-69. [Google Scholar]
[47]. Faivre S, Kroemer G, Raymond E, Current development of MTOR inhibitors as anticancer agentsNat Rev Drug Discov 2006 5:671-88. [Google Scholar]
[48]. Downward J, Targetting Ras signalling pathways in cancer therapyNat Rev Cancer 2003 3:11-22. [Google Scholar]
[49]. Mesa RA, Tipifarnib: farnesyl transferase inhibition at a crossroadsExpert Rev Anticancer Ther 2006 6:313-19. [Google Scholar]
[50]. Liu M, Bryant MS, Chen J, Antitumour activity of SCH 66336, an orally bioavailable tricyclic inhibitor of farnesyl protein transferase, in human tumour xenograft models and wap-ras transgenic miceCancer Res 1998 58:4947-56. [Google Scholar]
[51]. Zhang Y, Griffith EC, Sage J, Jacks T, Liu JO, Cell cycle inhibition by the anti- angiogenic agent TNP – 470 is mediated by p53 and p21WAF1/ CIP1Proc Nat Acad Sci USA 2000 97:6427-32. [Google Scholar]
[52]. Franks ME, Macpherson GR, Figg WD, ThalidomideLancet 2004 363:1802-11. [Google Scholar]